Session Information
Date: Sunday, November 17, 2024
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
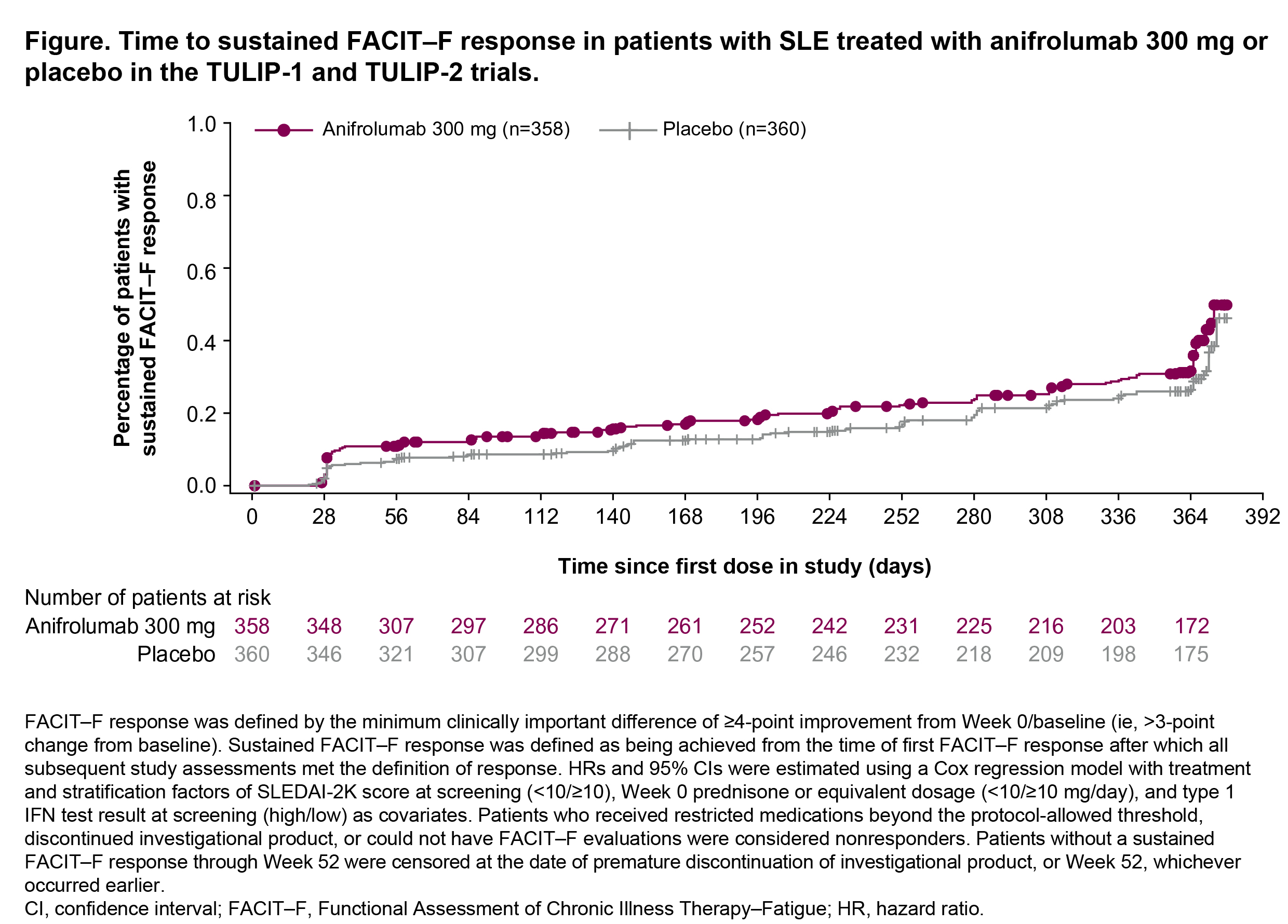
Background/Purpose: Fatigue is highly prevalent and severely affects health-related quality of life (HRQoL) in patients with SLE.1,2 We previously demonstrated that, compared with placebo, a numerically greater proportion of patients with moderate to severe SLE receiving treatment with the type I IFN receptor antibody anifrolumab in the phase 3 TULIP-1 and TULIP-2 trials reported improvements in fatigue at Week 52.3 However, fatigue fluctuates over time,2 and treatments that enable sustained improvements are needed. In this post hoc analysis of the TULIP-1/-2 trials, we assessed sustained fatigue response in patients receiving anifrolumab versus placebo.
Methods: Adults with moderate to severe SLE despite standard therapy were randomized in the double-blind, 52-week TULIP-1/-2 trials (NCT02446912, NCT02446899) to receive intravenous anifrolumab 300 mg or placebo every 4 weeks (Q4W).3 Patients completed the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT–F) questionnaire Q4W to provide a measurement of their fatigue and its impact on daily life. Based on the minimum clinically important difference, FACIT–F response was defined as a ≥4-point improvement from Week 0 assessment.3 Sustained FACIT–F response was defined as being achieved from the time of first FACIT–F response after which all subsequent assessments met the definition of response. Hazard ratios (HR) and 95% confidence intervals (CI) were estimated using a Cox regression model with treatment and stratification factors of SLEDAI-2K score at screening, Week 0 prednisone or equivalent dosage, and type 1 IFN test result at screening as covariates. Patients who received restricted medications beyond protocol-allowed thresholds, discontinued investigational product, or could not be evaluated for FACIT–F were considered nonresponders. P-values are nominal.
Results: Similar proportions of patients achieved a FACIT–F response at any time during the trials with anifrolumab and placebo (71.8% [257/358] and 71.1% [256/360]; HR 0.99, 95% CI 0.83–1.18). A numerically higher percentage of patients achieved a FACIT–F response at Week 52 with anifrolumab versus placebo (34.1% [122/358] vs 26.1% [94/360]). Patients on anifrolumab achieved sustained response earlier in the trials versus placebo (HR 1.30, 95% CI 1.00–1.71, nominal P=0.0542), with the numerical difference between the groups favoring anifrolumab versus placebo maintained from Week 4 to Week 52 (Figure).
Conclusion: Considering the severe impact of fatigue on HRQoL in patients with SLE1, it is important that improvements in fatigue be not only temporarily achieved but sustained. Though the proportions of patients achieving FACIT-F response during the trials did not differ with treatment, a numerically higher proportion of patients treated with anifrolumab achieved a FACIT–F response early in the trials that was sustained over time compared with placebo. Our findings are suggestive that anifrolumab treatment may provide longer-term improvements in fatigue and that further research is warranted.
References:
1. Elefante E. RMD Open. 2020;6(1):e001133.
2. Kawka L. J Clin Med. 2021;10(17):3996.
3. Strand V. Lancet Rheumatol. 2022;4(3):e198–207.
To cite this abstract in AMA style:
Strand V, Kalunian K, Bruce I, Seo C, Lee K, Knagenhjelm J, Lindholm C. Sustained Functional Assessment of Chronic Illness Therapy–Fatigue Response in Patients with SLE Receiving Anifrolumab Alongside Standard Therapy [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/sustained-functional-assessment-of-chronic-illness-therapy-fatigue-response-in-patients-with-sle-receiving-anifrolumab-alongside-standard-therapy/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sustained-functional-assessment-of-chronic-illness-therapy-fatigue-response-in-patients-with-sle-receiving-anifrolumab-alongside-standard-therapy/