Session Information
Date: Tuesday, November 9, 2021
Title: RA – Treatments Poster III: RA Treatments & Their Safety (1674–1710)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Sustained clinical remission (REM) is the primary treatment goal for patients with rheumatoid arthritis (RA), with low disease activity (LDA) being an appropriate target for treatment-refractory patients.1,2 This analysis evaluated the sustainability of response to the JAK inhibitor, upadacitinib (UPA) 15 mg once daily (QD), among patients with prior inadequate response or intolerance to biologic DMARDs.
Methods: Data come from the 12-week, phase 3 randomized, placebo (PBO)-controlled SELECT-BEYOND trial of UPA 15 mg or 30 mg QD in patients with moderate to severe RA on stable background conventional synthetic (cs) DMARDs. Initiation, change, or discontinuation of background RA medications, including ≤2 csDMARDs, was allowed starting at Week 24. Patients completing the 12-week trial were able to enter a long-term extension of up to 5 yrs with all PBO patients switching to UPA.3 This post hoc analysis evaluated REM (CDAI ≤2.8; SDAI ≤3.3), LDA (CDAI≤10; SDAI≤11), and DAS28(CRP) < 2.6/≤3.2 at first occurrence of response before Week 60, as well as at 3, 6, and 12 months following initial response in patients randomized to UPA 15 mg. For those patients who achieved REM/LDA, Kaplan-Meier was used to define the time from when the response was first achieved to the earliest date at which the response was lost at two consecutive visits or discontinuation of study drug. The predictive ability of time to REM/LDA was evaluated using Harrell’s concordance (c)-index (range: 0 to 1, where 0.5 indicates a model that is no better at predicting an outcome than random chance). The date of the last follow up was 16 April 2018, when all patients had reached the Week 60 visit. Non-responder imputation was used for missing data. Only data from the approved 15 mg dosage are reported here.
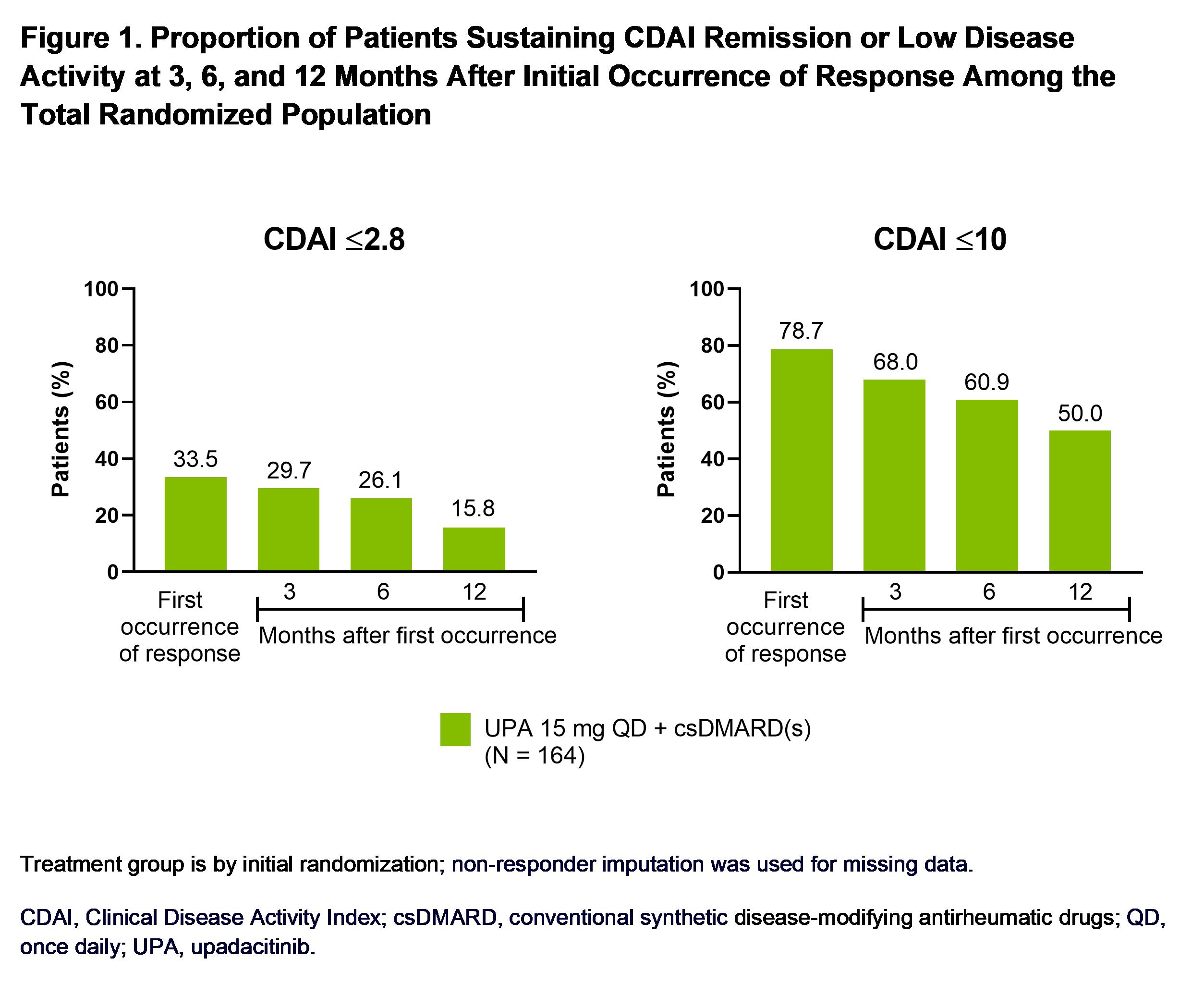
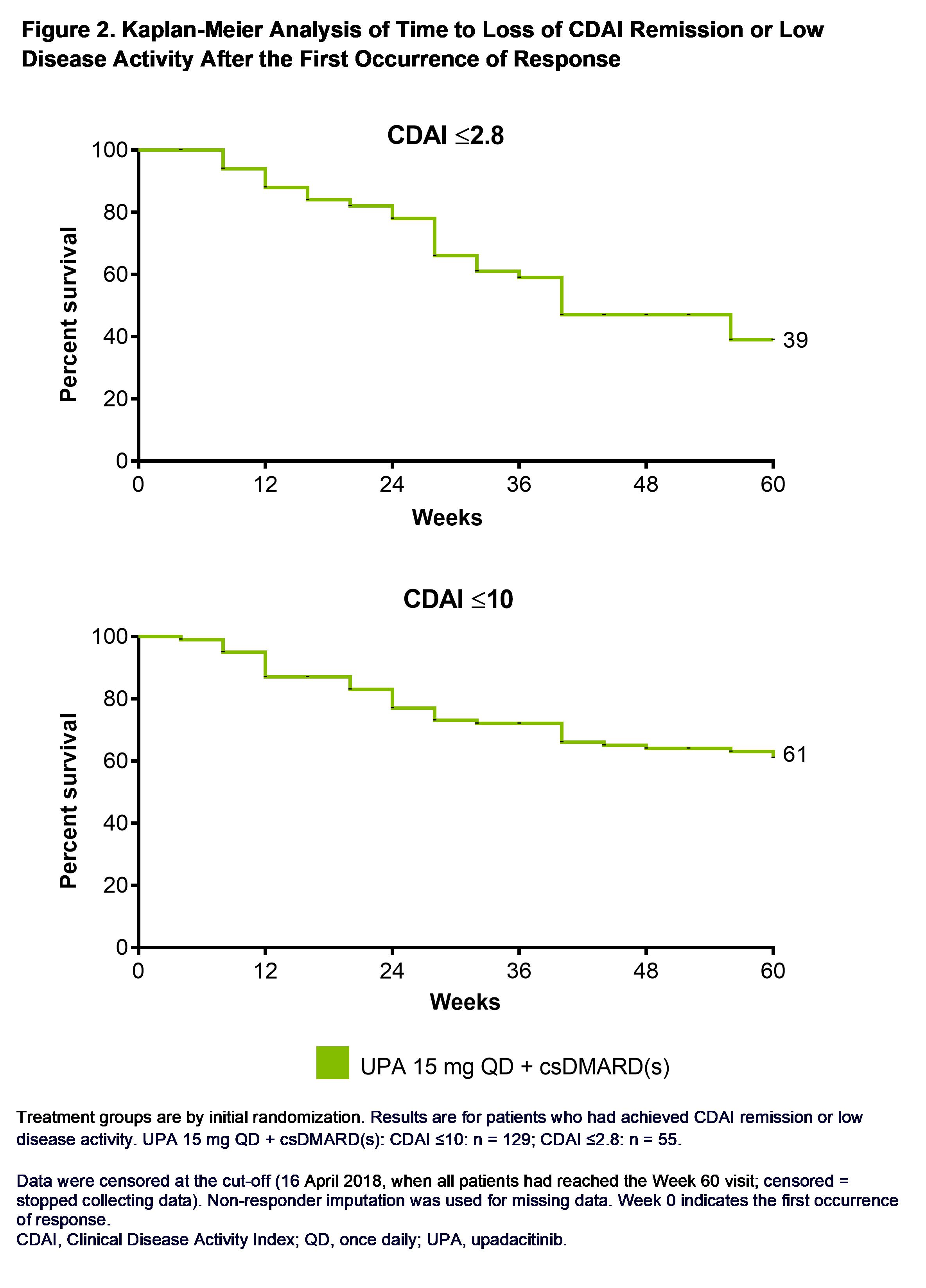
Results: In patients with active RA despite prior treatment with at least one bDMARD, 34% and 79% of those receiving UPA 15 mg + background csDMARD(s) achieved CDAI REM or LDA through Week 60, respectively. Sustained CDAI REM was attained by 30%, 26%, and 16% of patients randomized to UPA at 3, 6, and 12 months post initial response, while CDAI LDA was achieved by 68%, 61%, and 50% of patients during the same time points (Figure 1). Time to initial clinical response weakly predicted sustained REM but did not predict sustained LDA, with a c-index (95% CI) of 0.62 (0.49, 0.74) and 0.52 (0.44, 0.61), respectively. Through the last follow-up visit at Week 60, 39/61% of patients on UPA remained in CDAI REM/LDA (Figure 2). Of those who lost CDAI REM, 58% remained in CDAI LDA, and 22% recaptured REM by the cut-off date; 18% of patients who lost CDAI LDA recaptured response. Similar results were observed for REM and LDA based on SDAI and for DAS28(CRP) < 2.6/≤3.2.
Conclusion: Among patients with inadequate response or intolerance to bDMARDs, over three-quarters on UPA 15 mg achieved CDAI LDA, a relevant therapeutic target for these treatment-refractory patients, and nearly two-thirds of those maintained this response through 60 weeks. Additionally, about one-third of UPA-treated patients attained CDAI REM and maintained that response over 60 weeks.
1. Smolen et al. Ann Rheum Dis 2020;79:685–99.
2. Singh et al. Arthritis Rheumatol 2016;68:1–26.
3. Genovese, et al. Lancet 2018;391:2513–24.
To cite this abstract in AMA style:
van Vollenhoven R, Hall S, Wells A, Meerwein S, Song Y, Suboticki J, Fleischmann R. Sustainability of Response to Upadacitinib Among Patients with Active Rheumatoid Arthritis Refractory to Biological Disease-Modifying Anti-Rheumatic Drugs [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/sustainability-of-response-to-upadacitinib-among-patients-with-active-rheumatoid-arthritis-refractory-to-biological-disease-modifying-anti-rheumatic-drugs/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sustainability-of-response-to-upadacitinib-among-patients-with-active-rheumatoid-arthritis-refractory-to-biological-disease-modifying-anti-rheumatic-drugs/