Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Upadacitinib 15 mg (UPA), an oral Janus kinase inhibitor, has shown efficacy and tolerability through 14 weeks in patients (pts) with active radiographic axial SpA (also known as AS), including biological DMARD (bDMARD)-naive pts and pts with intolerance or inadequate response [IR] to bDMARDs, and in pts with non-radiographic axial SpA (nr‑axSpA).1‑3 Sustained clinical response is a crucial treatment target. We evaluated the 52‑week sustainability of clinical responses in pts with active AS and nr-axSpA from the SELECT-AXIS 1 (NCT03178487) and SELECT-AXIS 2 (NCT04169373) trials who achieved week 14 treatment responses.
Methods: This post hoc analysis included adult pts enrolled in SELECT-AXIS 1 (bDMARD-naive AS) or SELECT-AXIS 2 (comprises 2 studies conducted under a master protocol: 1 in pts with bDMARD-IR AS and 1 in pts with nr‑axSpA). Efficacy measures included Assessment of SpA international Society 40 (ASAS40) response, ASAS partial remission (ASAS‑PR), AS Disease Activity Score (ASDAS) status (inactive disease [ID] and low disease activity [LDA]) and improvement (clinically important or major improvement), achievement of ≤2.0 score on numerical rating scale (NRS) and minimal clinically important difference (MCID) in total and nocturnal back pain,4 and achievement of ≤1.5 score and MCID in BASFI.5,6 Durability of response was assessed at week 52 in pts who achieved a response in each efficacy endpoint at week 14 with UPA. No imputation of missing data was performed. The 95% CIs for durability of response were based on Wald limits without continuity correction.
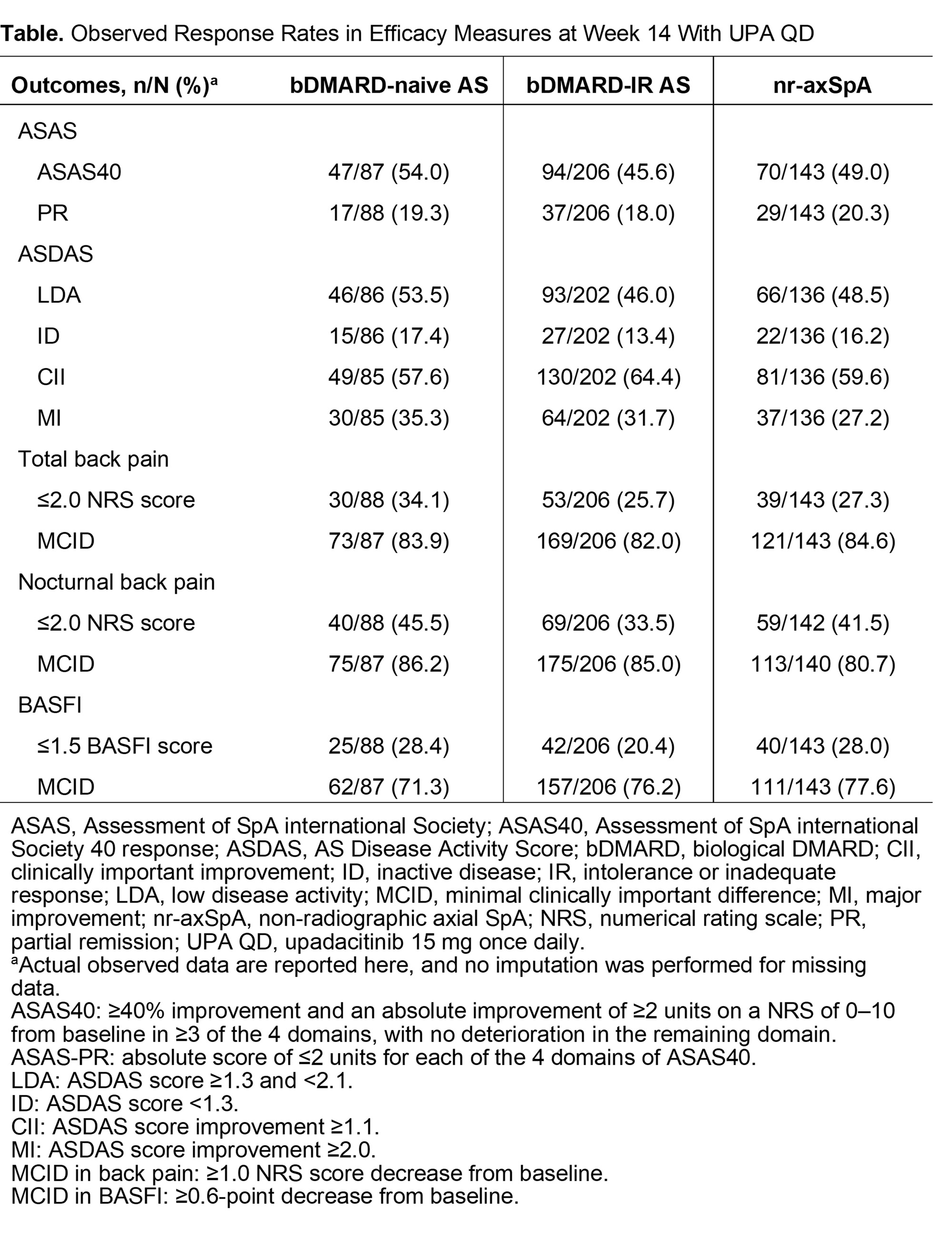
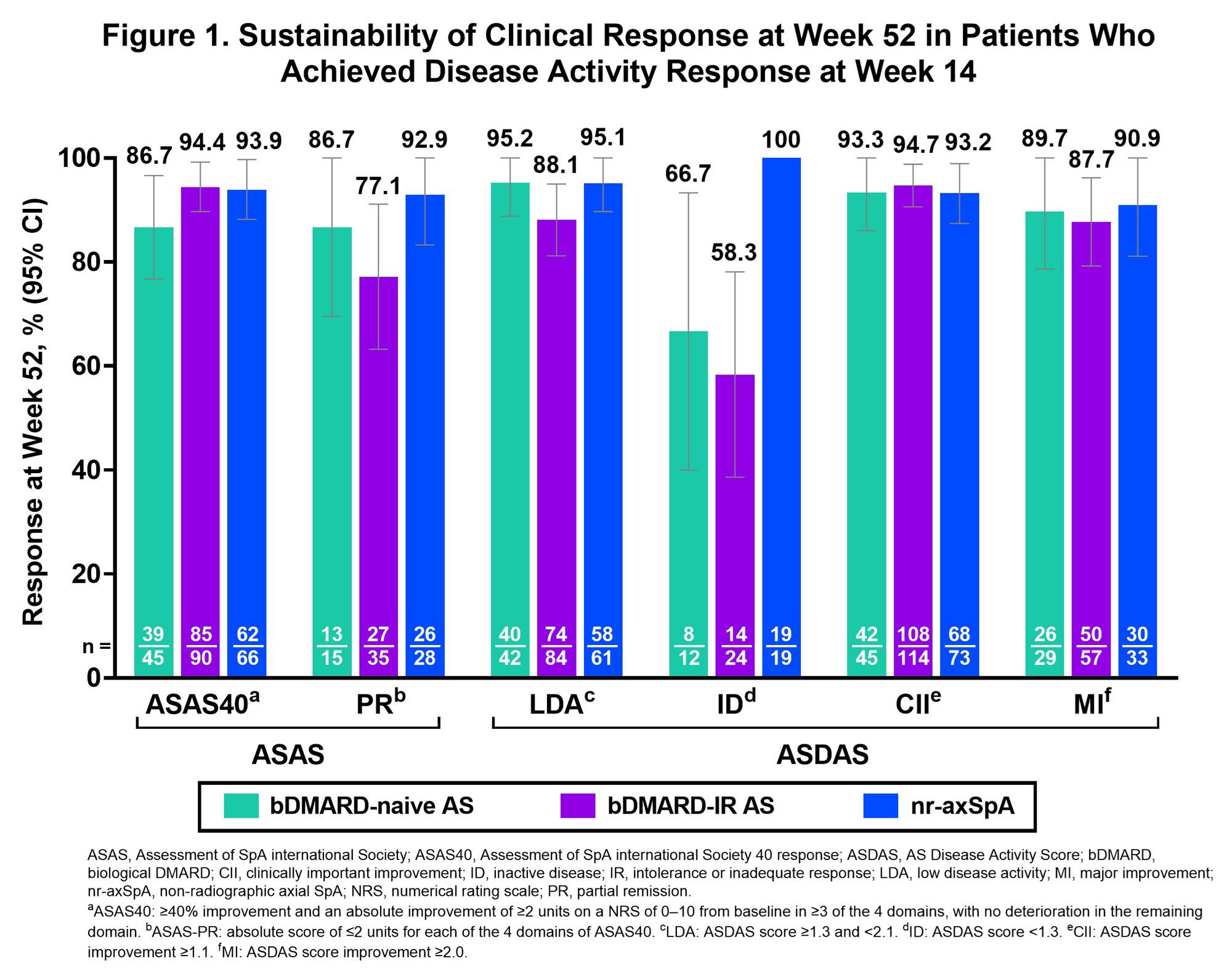
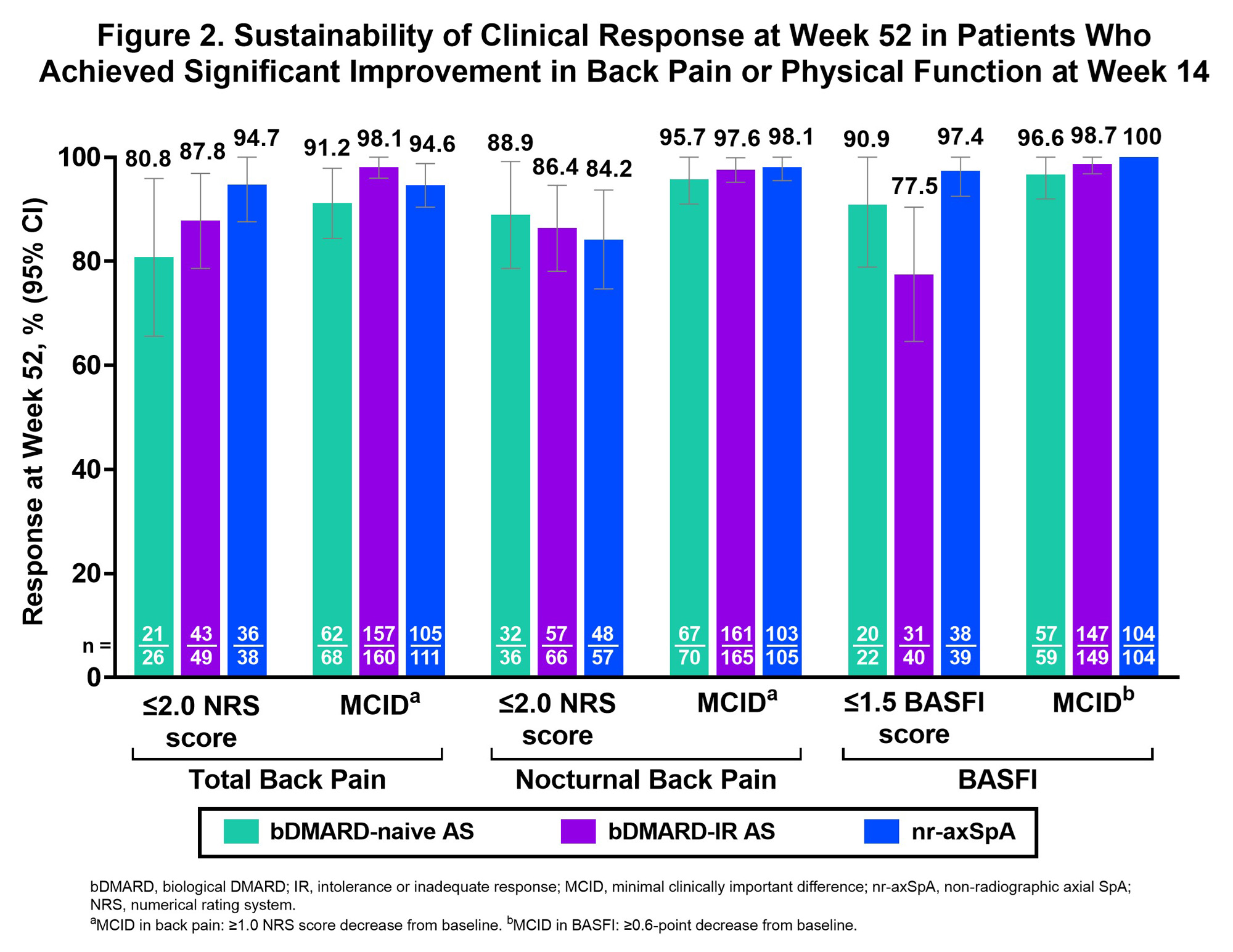
Results: This analysis included 460 pts (bDMARD-naive AS, n=93; bDMARD-IR AS, n=211; nr-axSpA, n=156). In pts who achieved ASAS40 response at week 14 (Table), most sustained this response at week 52 (bDMARD-naive AS, 86.7%; bDMARD-IR AS, 94.4%; nr‑axSpA, 93.9%; Fig 1). Similar results occurred in other disease activity measures with pts sustaining effects at week 52: ASAS PR (77.1%–92.9% across pt groups), ASDAS-LDA (88.1%–95.2%), ASDAS-ID (58.3%–100%), ASDAS-CII (93.2%–94.7%), and ASDAS-MI (87.7%–90.9%). At week 52, sustained treatment effects were also observed on back pain (≤2.0 NRS score: total [80.8%–94.7%], nocturnal [84.2%–88.9%]; MCID: total [91.2%–98.1%]; nocturnal [95.7%–98.1%]) and on BASFI (score ≤1.5: 77.5%–97.4%; MCID: 96.6%–100%; Fig 2). In the bDMARD-IR AS group, sustainability of more stringent outcomes (ASAS PR, ASDAS-LDA, ASDAS-ID, and BASFI score ≤1.5) were numerically lower vs other pt groups (Fig 1–2).
Conclusion: UPA-treated pts with a clinical response at week 14 experienced a sustained response in improvements in disease activity, back pain, and physical function at week 52, supporting the long-term efficacy of UPA across the entire axSpA spectrum.
1. van der Heijde D, et al. Ann Rheum Dis. 2022;81:1515–23.
2. van der Heijde D, et al. Lancet. 2019;394:2108–17.
3. Deodhar A, et al. Lancet. 2022;400:369–79.
4. Salaffi F, et al. Eur J Pain. 2004;8:283–91.
5. Wariaghli G, et al. BMC Musculoskelet Disord. 2012;13:40.
6. Kviatkovsky MJ, et al. J Rheumatol. 2016;43:1680–6.
To cite this abstract in AMA style:
Navarro-Compán V, Gensler L, Rudwaleit M, Ganz F, Chen S, Stigler J, Schmagel A, Baraliakos X. Sustainability of Clinical Response at Week 52 to Upadacitinib Among Patients with Axial SpA: Data from the SELECT-AXIS 1 and SELECT-AXIS 2 Trials [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/sustainability-of-clinical-response-at-week-52-to-upadacitinib-among-patients-with-axial-spa-data-from-the-select-axis-1-and-select-axis-2-trials/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sustainability-of-clinical-response-at-week-52-to-upadacitinib-among-patients-with-axial-spa-data-from-the-select-axis-1-and-select-axis-2-trials/