Session Information
Date: Tuesday, November 19, 2024
Title: Abstracts: RA – Treatment II: Refining Use of Established Therapies
Session Type: Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: For patients (pts) early in their RA disease course, with a clinical profile characterized by inadequate response to MTX (MTX-IR), high titers of ACPA (as measured by anti-CCP2 antibodies) and RF (dual seropositivity) combined with the presence of the shared epitope (SE) HLA risk allele may be predictive of an enhanced response to treatment (tx) with abatacept (ABA) compared with adalimumab (ADA).1-3
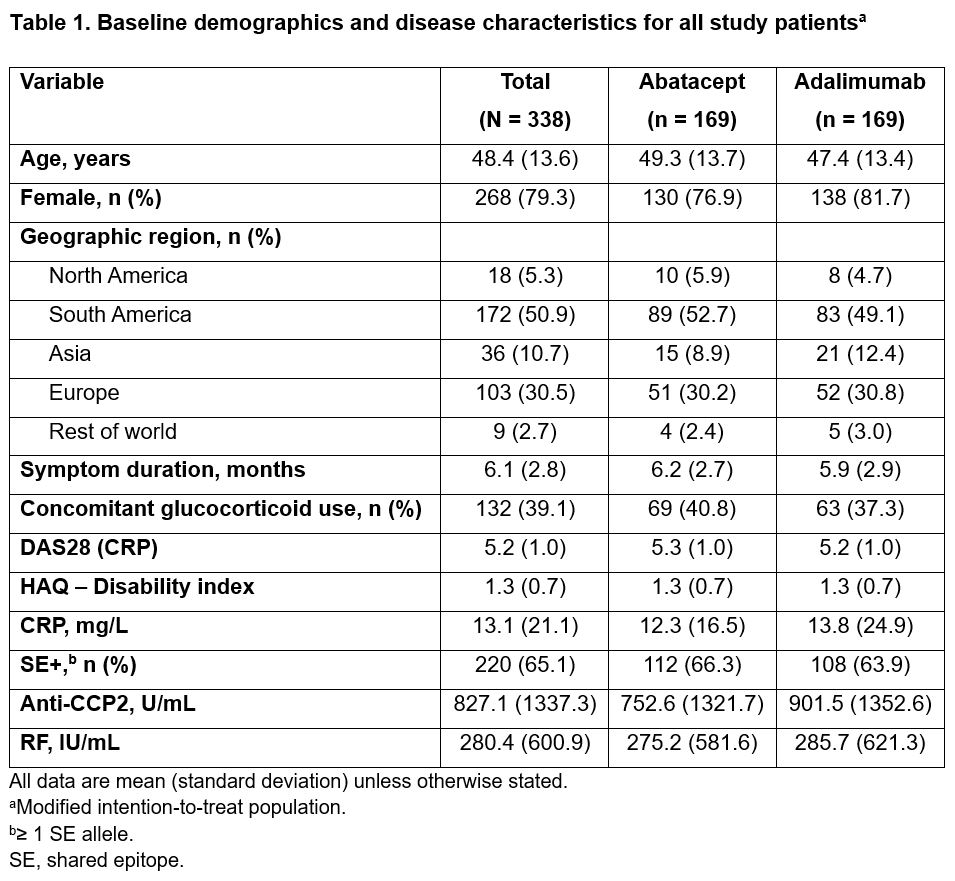
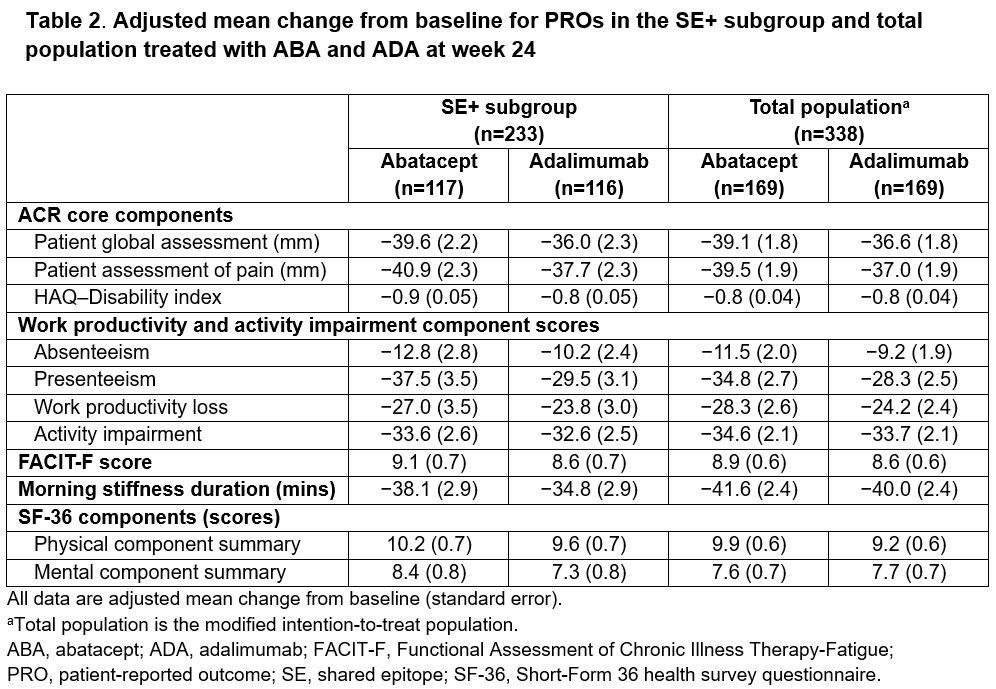
Methods: The AMPLIFIED trial is a phase 3, randomized, single-blind study evaluating the superiority of ABA compared with ADA in pts who met the ACR/EULAR 2010 criteria for RA (NCT04909801). Pts with (+) or without (−) the SE HLA class II risk alleles were enrolled (the study was powered for the SE+ subset). All pts were on stable background MTX (goal, 15–25 mg/week). No other prior DMARD use was allowed. The primary endpoint was 50% improvement in ACR criteria (ACR50) at week 24 in pts who were SE+. Secondary outcomes included DAS28 (CRP) < 2.6, antibody titers, and adverse events (AEs) at week 24. Post hoc analysis of results stratified by anti-CCP2 titers was performed. Additional analysis compared outcomes, including a range of patient-reported outcomes (PROs), between ABA and ADA tx in the SE+ subgroup and total study population.
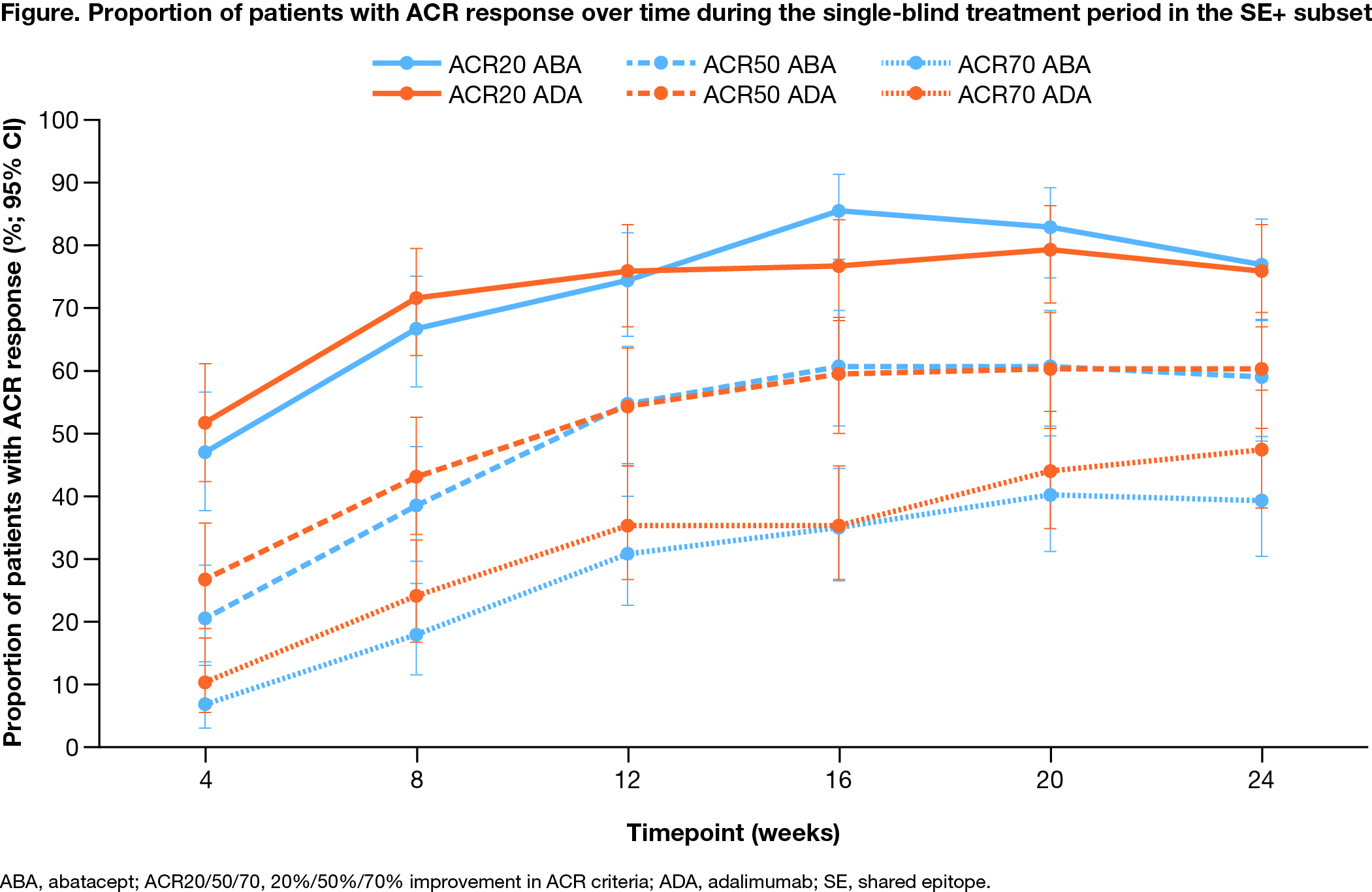
Results: A majority of the total study population (95.9%) completed the single-blind tx period (24 weeks). Baseline demographic and disease characteristics were balanced between tx groups (Table 1). Mean baseline anti-CCP2 titers were high and ~65% of pts were SE+. The study failed to meet the primary endpoint: ACR50 rate in the SE+ subset at week 24 was 59% with ABA and 60% with ADA; adjusted odds ratio (aOR; 95% CI): 1.0 (0.6–1.6); P = 0.9. The ACR50 rate across the study population was also comparable across txs (59% vs 64% with ABA vs ADA; aOR [95% CI]: 0.8 [0.5–1.3]). DAS28 (CRP) < 2.6 was 44% with ABA vs 47% with ADA; aOR (95% CI): 0.9 (0.5–1.5). ACR responses over time were comparable between the arms in onset and magnitude (Figure). PROs were similar between ADA and ABA in the SE+ subgroup and total population (Table 2). Mean decrease from baseline to week 24 in autoantibody titers was more notable with ABA vs ADA (anti-CCP2: −196 vs −109 U/mL; RF: −121 vs −27 IU/mL), while the change in CRP was comparable (−6.2 vs −5.7 mg/L) at week 24. Pts with the highest anti-CCP2 levels at baseline trended toward better tx response with ABA compared with ADA. Both drugs were well tolerated with comparable rates of AEs (ABA, 58.0%; ADA, 59.2%) and serious AEs (ABA, 2.4%; ADA, 3.6%). No serious infections were seen with ABA, with 3 seen with ADA. COVID-19 was seen in 12.7% of all pts (all mild). AEs of special interest were similar across ABA and ADA tx groups.
Conclusion: The AMPLIFIED trial failed to confirm the value of autoantibody and SE positivity for predicting response to tx with ABA vs ADA, seen in the prior pilot study.3 Most pts with early RA tolerated and responded well to both ABA and ADA across all measures, including a broad range of PROs.
References
1. Weinblatt ME, et al. Arthritis Rheum 2013;65(1):28–38.
2. Sokolove J, et al. Ann Rheum Dis 2016;75(4):709–14.
3. Rigby W, et al. Arthritis Res Ther 2021;23:245.
Medical writing
Megan Murchie, MRes (Caudex), funded by Bristol Myers Squibb.
To cite this abstract in AMA style:
Weinblatt M, Emery P, Bykerk V, Cope A, Burmester G, Tanaka Y, Citera G, Nash P, Dornic Q, Kelly S, Maldonado M. Subcutaneous Abatacept vs Adalimumab Head-to-Head Comparison in Adults with Early, Dual Seropositive Rheumatoid Arthritis, Positive for the Shared Epitope HLA Class II Risk Alleles, and an Inadequate Response to Methotrexate: Results from a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/subcutaneous-abatacept-vs-adalimumab-head-to-head-comparison-in-adults-with-early-dual-seropositive-rheumatoid-arthritis-positive-for-the-shared-epitope-hla-class-ii-risk-alleles-and-an-inadequate/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/subcutaneous-abatacept-vs-adalimumab-head-to-head-comparison-in-adults-with-early-dual-seropositive-rheumatoid-arthritis-positive-for-the-shared-epitope-hla-class-ii-risk-alleles-and-an-inadequate/