Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Sprifermin, a recombinant human fibroblast growth factor 18, is currently being investigated as a potential disease-modifying OA drug. Sprifermin treatment leads to a dose-dependent increase in femorotibial cartilage thickness, as well as medial and lateral compartment cartilage, over two years1. The aim of this post-hoc analysis was to evaluate the potential effects of sprifermin on additional knee structural endpoints, based on semi-quantitative MRI assessment over 24 months.
1Hochberg MC, et al. Arthritis Rheumatol. 2017; 69 (suppl 10).
Methods: Patients aged 40–85 years with symptomatic radiographic primary femorotibial OA according to ACR criteria, Kellgren-Lawrence Grade 2 or 3, and medial minimum joint space width ≥2.5 mm in the target knee were randomized (1:1:1:1:1) to receive double-blinded sprifermin (30 μg or 100 μg) or placebo, administered as three weekly intra-articular injections in 6- or 12-month cycles. 1.5 or 3 Tesla MRIs were acquired at baseline, 6, 12, 18 and 24-month follow-up visits using a standard protocol (NCT01033994).
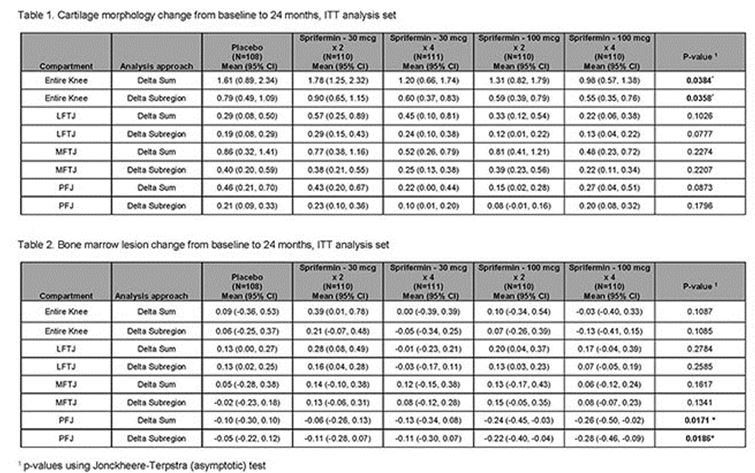
MRIs were read using the Whole-Organ Magnetic Resonance Imaging Score (WORMS) system by three trained musculoskeletal radiologists at baseline, 12 and 24 months. Analyses of all sprifermin and placebo arms included multiple MRI-defined OA features and multi-dimensional assessments: (a) delta-subregional approach (the difference in the number of subregions with worsening as compared to improvement) and (b) delta-sum approach (absolute scores of all subregions). Analyses were performed on a whole knee level and separately for medial, lateral, and patellofemoral compartments. To test for potential dose-response effects, the Jonckheere-Terpstra (asymptotic) test was used. P-values were not adjusted for multiple testing.
Results: In total, 549 patients were included. A dose-dependent treatment effect for sprifermin on cartilage morphology (i.e., less worsening of cartilage damage) was observed for the entire knee from baseline to 24 months using both delta sum and delta subregion approaches (Table 1). For bone marrow lesions (BMLs), a dose-dependent treatment effect (improvement of BMLs) was observed from baseline to 24 months for the patello-femoral joint, using both delta sum and delta subregion approaches, but not in the other compartments (Table 2). No significant changes from baseline to 24-months were reported in Hoffa-synovitis, effusion-synovitis, menisci, or osteophytes.
Conclusion: Sprifermin has a positive effect on cartilage morphology based on semi-quantitative assessment, in addition to the previously reported effect on cartilage thickness based on 3D morphometry. Sprifermin was also associated with BML improvement in the patello-femoral joint. There were no significant effects associated with sprifermin on the other joint tissues assessed, and no additional safety concerns.
To cite this abstract in AMA style:
Guermazi A, Kraines J, Aydemir A, Wax S, Crema M, Hochberg MC, Roemer F. Structural Effects of Intra-Articular Sprifermin in Symptomatic Radiographic Knee Osteoarthritis: A Post-Hoc Analysis of Cartilage Morphology over the 2-Year Treatment-Period of a 5-Year Randomized, Placebo-Controlled, Phase II Study [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/structural-effects-of-intra-articular-sprifermin-in-symptomatic-radiographic-knee-osteoarthritis-a-post-hoc-analysis-of-cartilage-morphology-over-the-2-year-treatment-period-of-a-5-year-randomized-p/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/structural-effects-of-intra-articular-sprifermin-in-symptomatic-radiographic-knee-osteoarthritis-a-post-hoc-analysis-of-cartilage-morphology-over-the-2-year-treatment-period-of-a-5-year-randomized-p/