Session Information
Date: Sunday, October 26, 2025
Title: (0280–0305) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Recent progress has been made in developing myositis outcome assessments, response and classification criteria, and consensus in the design and conduct of clinical trials through the International Myositis Assessment and Clinical Studies Group (IMACS). However, critical deficiencies remain for data standardization across myositis registries. While the National Institute of Health (NIH) Common Data Elements (CDEs) Repository has been developed to facilitate consistent data collection and data sharing, few myositis-specific or autoimmune disease CDEs currently exist. Here, we developed CDEs for myositis core set activity measures (CSMs) using novel data science strategies to support myositis research interoperability.
Methods: Dictionaries of several myositis registries were examined to understand how myositis CSMs are captured across databases. We used the Linked data Modeling Language (LinkML), an open-source data modeling framework for creating schemas for structured, linked data to develop computable CDEs. Information and metadata of CDEs were captured in a machine-readable format that leverages existing standards for data standardization and reuse. The Observational Health Data Science and Informatics (OHDSI) application Athena-OHDSI Vocabularies Repository (Odysseus Data Services, Inc.) was utilized to map CDEs to the Observational Medical Outcomes Partnership (OMOP) vocabulary. After development of draft CDEs for myositis CSMs, a hybrid conference was held to discuss results, reach consensus on the coding of myositis CSM CDEs, and to prioritize additional forms for development of CDEs. An international panel of 13 myositis experts and 11 discussants, including adult/pediatric rheumatologists, neurologists, dermatologists, physical therapists, and patients/patient advocates, attended the consensus conference. The Delphi method and modified nominal group technique were used in tandem as consensus methods for each CDE. Comments from participants were received via an open survey on REDCap and discussed, followed by electronic voting to reach consensus. Participants also ranked additional myositis measures for future CDE development.
Results: A standardized workflow has been established for creation of CDEs in myositis and other autoimmune diseases (Figure). CDEs for all myositis CSMs were drafted, except CHQ-P50 due to its restrictive licensing. After receiving several comments on each CDE and holding discussions to improve the coding of the CSM CDEs, 100% agreement among participants was reached for all CDEs (Table 1). The prioritized measures for future CDEs included IIM Response, Classification and Flare Criteria, damage measures, task-oriented physical function measures, and patient-reported outcome measures via PROMIS instruments among others (Table 2).
Conclusion: Leveraging broad multispecialty expertise in myositis and patient communities through IMACS and data science expertise of the National Library of Medicine (NLM), the first myositis-specific CDEs have been developed to accelerate the ability to conduct interoperable myositis clinical studies and therapeutic trials.
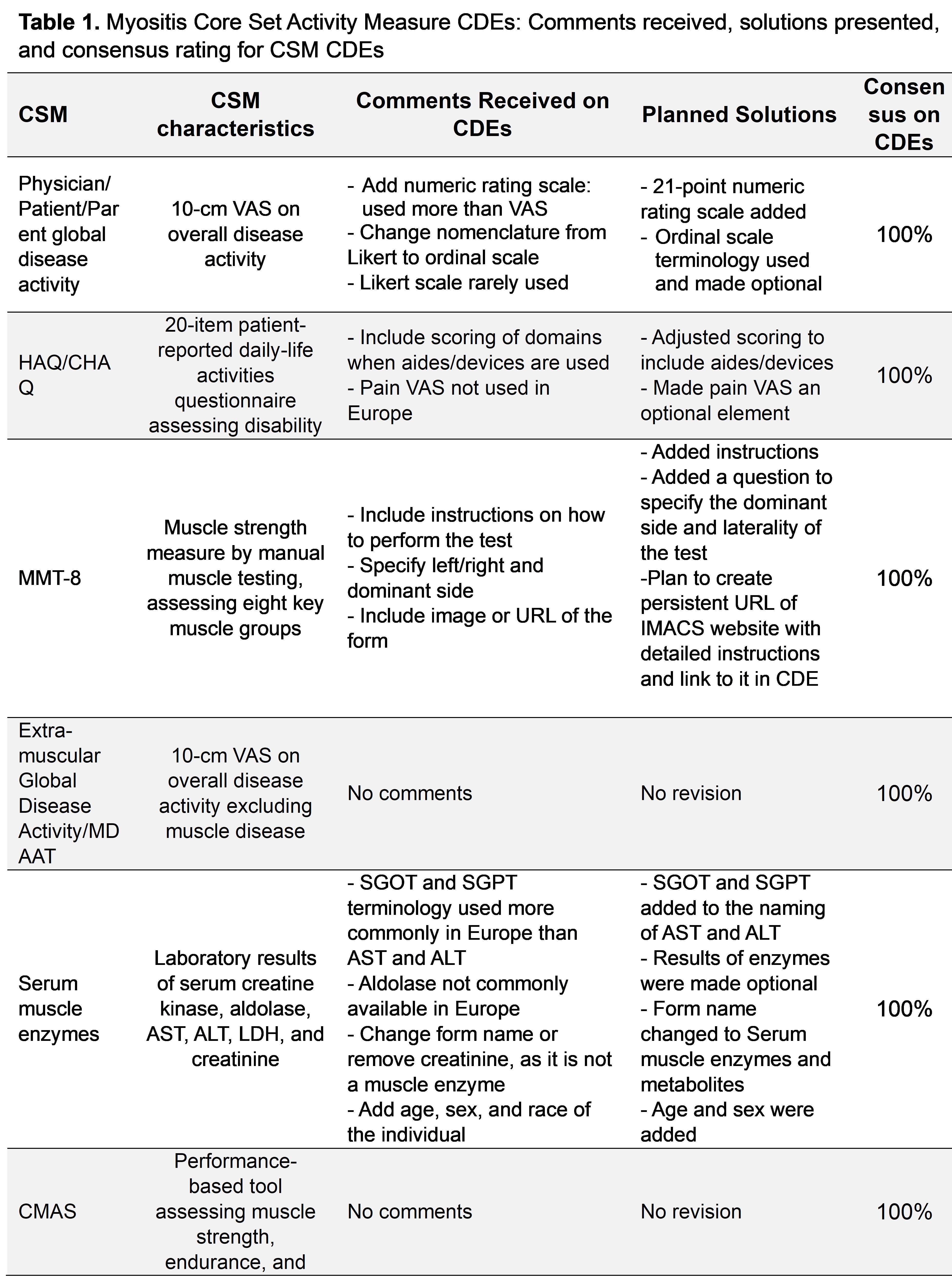 Figure 1. Flow diagram of the project steps.
Figure 1. Flow diagram of the project steps.
To cite this abstract in AMA style:
Saygin D, Diller M, Surampudi V, Bodkin M, Farhadi P, Schiffenbauer A, Kessel A, Mecoli C, Aggarwal R, Alexanderson H, Best M, Benveniste O, Chinoy H, Feldman B, Kobert L, Lubinus M, McCann L, V. Oddis C, Ruperto N, Schmidt J, Werth V, Bartels C, Kim H, Mammen A, Paik J, Werner E, de Groot I, Machado P, Kim S, Mozaffar T, Huber A, Ravelli A, Scheuermann R, Rider L. Standardized Interoperable Data Collection for Myositis Research: Developing Common Data Elements for Myositis Disease Activity Core Set Measures [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/standardized-interoperable-data-collection-for-myositis-research-developing-common-data-elements-for-myositis-disease-activity-core-set-measures/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/standardized-interoperable-data-collection-for-myositis-research-developing-common-data-elements-for-myositis-disease-activity-core-set-measures/

.jpg)
.jpg)