Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Synovial fluid (SF) of RA patients contains unique SPP1+ macrophages that drive pathogenesis by activating fibroblast-like synoviocytes (FLS). Single-cell RNA sequencing (scRNA-seq) data from SF, synovial tissue (ST), and peripheral blood of same RA patient shows SPP1+ macrophages are enriched in SF and ST but absent in peripheral blood. This limits research on these cells. Previous studies focused on RA patients and lack research on the distribution and functional characteristics of Spp1+ macrophages in animal models. This study explores the distribution and function of Spp1+ macrophages in collagen-induced arthritis (CIA) mice, addressing clinical sample limitations and providing insights into RA pathogenesis and therapeutic strategies.
Methods: CIA mouse model was established, and scRNA-seq was performed on joints from CIA and control mice. Sequencing data was annotated and clustered to identify cell populations. Flow cytometry analyzed Spp1+ macrophage proportions in peripheral blood, spleen, and joints based on established gating strategies. Micro-computed tomography (Micro-CT) was used to assess bone metabolism parameters and their correlation with Spp1+ macrophage proportions.
Results: On day 42 post-immunization, CIA mice showed significant weight loss, higher clinical scores, severe synovial inflammation, and joint destruction compared to controls, confirming successful model induction (Fig. 1A-E). scRNA-seq of paws from one CIA and one control mouse revealed Spp1+ macrophages specifically in CIA joints, absent in controls (Fig. 1F-H). Flow cytometry showed higher Spp1+ macrophage proportions in CIA joints than in peripheral blood and spleen (Fig. 2B-C). Proportion of Spp1+ macrophages in the joints of CIA mice were significantly elevated compared to controls, with no differences in peripheral blood and spleen (Fig. 2E-F). Three-dimensional reconstruction of knee joints showed severe joint surface damage and bone erosion in CIA mice. Bone volume (BV)/tissue volume (TV), bone surface (BS)/TV, trabecular number (Tb.N), and trabecular sepamtion (Tb.Sp) analysis in the proximal tibia showed significant decreases in BV/TV, BS/TV, and Tb.N in CIA mice (Fig. 3A-C), while Tb.Sp was higher (Fig. 3D), indicating bone loss and osteoporosis. Spearman correlation analysis showed Spp1+ macrophage proportions in joints were negatively correlated with BV/TV, BS/TV, and Tb.N but positively correlated with Tb.Sp (Fig. 3E-H), suggesting a link between elevated Spp1+ macrophages and bone abnormalities in CIA mice.
Conclusion: This study reveals that Spp1+ macrophages are specific enrichment in CIA mouse joints and their association with abnormal bone metabolism. These findings offer new insights into RA pathogenesis, address clinical sample limitations, and provide experimental evidence for developing therapeutic strategies.
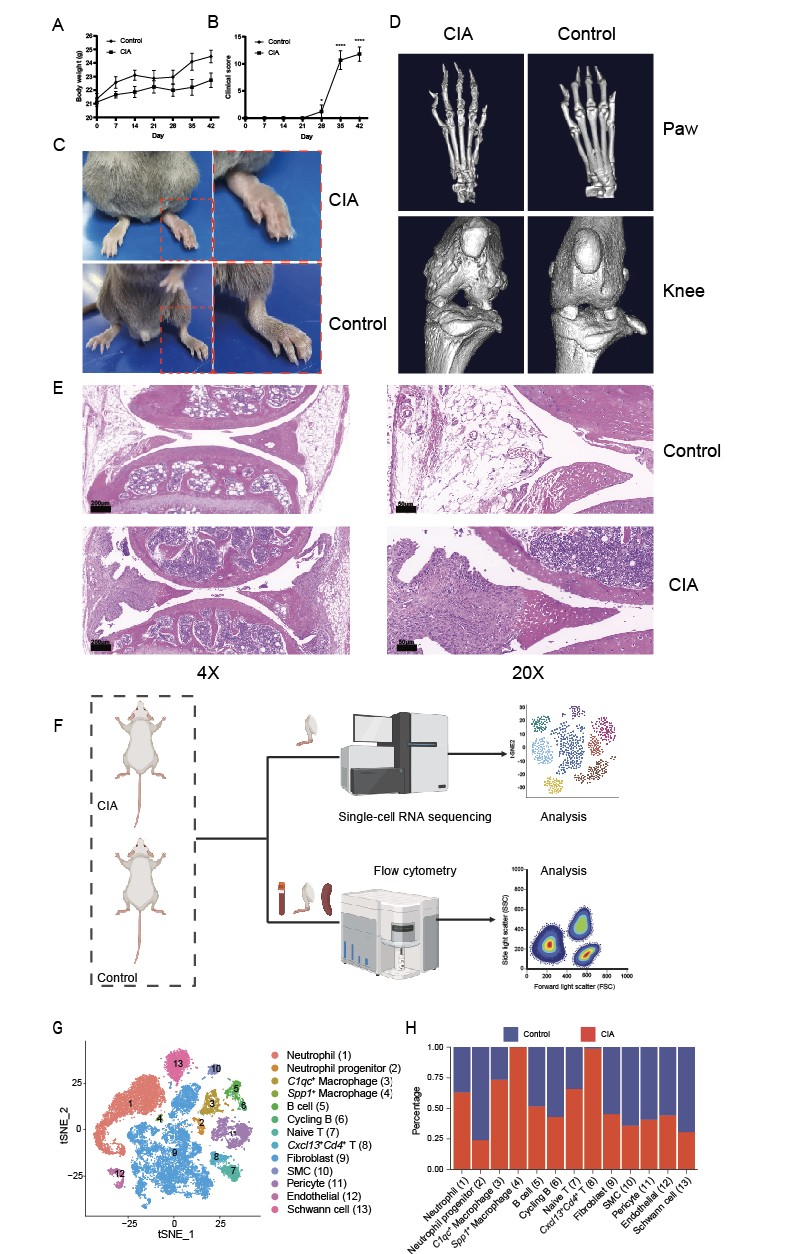 Fig. 1 Establishment of the CIA mouse model and single-cell sequencing analysis. A Body weight of CIA and control mice. B Clinical arthritis scores of CIA and control mice. C Hind paws of mice 42 days after primary immunization. D Representative micro-CT images of hind paws and knee joints. E Hematoxylin and Eosin staining of knee joint pathological sections. F Schematic diagram of the study workflow. G t-distributed stochastic neighbor embedding (t-SNE) plot showing single-cell transcriptional profiles of paws from CIA and control mice. H Proportion analysis of cell clusters in CIA versus control mice. *p < 0.05, ****p < 0.0001.
Fig. 1 Establishment of the CIA mouse model and single-cell sequencing analysis. A Body weight of CIA and control mice. B Clinical arthritis scores of CIA and control mice. C Hind paws of mice 42 days after primary immunization. D Representative micro-CT images of hind paws and knee joints. E Hematoxylin and Eosin staining of knee joint pathological sections. F Schematic diagram of the study workflow. G t-distributed stochastic neighbor embedding (t-SNE) plot showing single-cell transcriptional profiles of paws from CIA and control mice. H Proportion analysis of cell clusters in CIA versus control mice. *p < 0.05, ****p < 0.0001.
.jpg) Fig. 2 Flow cytometry analysis of peripheral blood, joints, and spleen in mice. A Gating strategy for identifying Spp1+ macrophages by flow cytometry. B Representative flow cytometry plots of Spp1+ macrophages in joints, peripheral blood, and spleen from collagen-induced arthritis (CIA) (n=9) and control (n=5) mice. C Quantitative analysis of the proportion of Spp1+ macrophages across different tissues. D Quantitative comparison of spp1+ macrophage proportions in joints between CIA and control mice. E Quantitative comparison of Spp1+ macrophage proportions in peripheral blood between CIA and control mice. F Quantitative comparison of Spp1+ macrophage proportions in spleen between CIA and control mice. n.s., not significant (p > 0.05); ***p < 0.001; ****p < 0.0001.
Fig. 2 Flow cytometry analysis of peripheral blood, joints, and spleen in mice. A Gating strategy for identifying Spp1+ macrophages by flow cytometry. B Representative flow cytometry plots of Spp1+ macrophages in joints, peripheral blood, and spleen from collagen-induced arthritis (CIA) (n=9) and control (n=5) mice. C Quantitative analysis of the proportion of Spp1+ macrophages across different tissues. D Quantitative comparison of spp1+ macrophage proportions in joints between CIA and control mice. E Quantitative comparison of Spp1+ macrophage proportions in peripheral blood between CIA and control mice. F Quantitative comparison of Spp1+ macrophage proportions in spleen between CIA and control mice. n.s., not significant (p > 0.05); ***p < 0.001; ****p < 0.0001.
.jpg) Fig. 3 Correlation analysis between bone metabolic parameters and Spp1+ macrophages. (A) Bone volume to tissue volume ratio (BV/TV), (B) bone surface to tissue volume ratio (BS/TV), (C) trabecular number (Tb.N), and (D) trabecular separation (Tb.Sp) in the proximal tibia of CIA (n=6) and control (n=5) mice. (E-H) Correlation analysis between the proportion of Spp1+ macrophages in joints and (E) BV/TV, (F) BS/TV, (G) Tb.N, and (H) Tb.Sp. *p < 0.05, **p < 0.01.
Fig. 3 Correlation analysis between bone metabolic parameters and Spp1+ macrophages. (A) Bone volume to tissue volume ratio (BV/TV), (B) bone surface to tissue volume ratio (BS/TV), (C) trabecular number (Tb.N), and (D) trabecular separation (Tb.Sp) in the proximal tibia of CIA (n=6) and control (n=5) mice. (E-H) Correlation analysis between the proportion of Spp1+ macrophages in joints and (E) BV/TV, (F) BS/TV, (G) Tb.N, and (H) Tb.Sp. *p < 0.05, **p < 0.01.
To cite this abstract in AMA style:
He C, Xia X, Xu H, Yin G, Xie Q. Spp1+ Macrophages Are Specifically Enriched in Arthritic Joints and Associated with Abnormal Bone Metabolism in Collagen-Induced Arthritis Mice [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/spp1-macrophages-are-specifically-enriched-in-arthritic-joints-and-associated-with-abnormal-bone-metabolism-in-collagen-induced-arthritis-mice/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/spp1-macrophages-are-specifically-enriched-in-arthritic-joints-and-associated-with-abnormal-bone-metabolism-in-collagen-induced-arthritis-mice/
