Session Information
Date: Monday, October 27, 2025
Session Type: Abstract Session
Session Time: 4:15PM-4:30PM
Background/Purpose: Psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA) patients are at increased risk for herpes zoster(HZ) due to impaired cell-mediated immunity associated with the underlying disease and therapies. Although the adjuvanted recombinant zoster vaccine (RZV, Shingrix®) has shown high efficacy and a favorable safety profile in immunocompetent individuals, its performance in PsA and axSpA patients under immunosuppressive treatment has not been evaluated and may hamper vaccination recommendations for these patients. The present study evaluates the humoral immunogenicity and safety of RZV in immunosuppressed PsA and axSpA patients compared to healthy controls (CG) and to investigate the possible impact of immunosuppressive conventional and/or biologic disease-modifying antirheumatic drugs (DMARDs) and disease activity on vaccine response.
Methods: This was a prospective, randomized, placebo-controlled clinical trial enrolled adult PsA and axSpA. Patients were randomized 1:1 to receive RZV (P1) and placebo(P2). A CG was included at a 2:1 (ARD:CG) ratio. Both CG and P1 groups received two intramuscular dose of the recombinant zoster vaccine (RZV) administered 6 weeks apart (Day 0 and Day 42) while P2 received placebo. After unblinding at Day 84, P2 participants received two doses of the vaccine. A positive humoral response was defined as an anti-gE antibody concentrations at least four times above the assay’s lower detection at baseline and 6 weeks after second dose. Geometric mean titres (GMTs) and factor increase (FI) were calculated. Safety was evaluated using standardized one-week diary and disease flares was assessed by the rheumatologist in each visit using validated disease indices/scores: BASDAI, ASDAS, PASI, DAPSA and MDA.
Results: A total of 185 SpA patients and 92 controls with similar sex distribution (37.8% vs. 38.0% female, p=0.974) and age (54.7+13.0 vs. 58.3+8.6 years, p=0.071) were enrolled. At D84 after vaccination, seroconversion (93.9% vs. 98.9%,p=0.037) and GMT [(8.9(7.6-10.4) vs. 13.5(11.6-15.7), p=0.001)] were significantly reduced in patients compared to CG. FI-GMT was similar between groups [(43.3(33.9-55.3) vs. 59.0(44.1-78.8), p=0.083)]. No factor was identified to contribute to decreased seroconversion in SpA patients (p >0.05). Disease safety evaluation revealed no increase in disease flares among patients who received P1 in comparison to patients who received P2 at D84 (11.8% vs. 11.3%,p=0.918) in the whole group or among patients with axSpA according to ASDAS-CRP (7.5% vs. 3.2%,p=0.627) or PsA according to the DAPSA (15.6% vs 16.3%,p=0.919) (Figure 1). RZV was well-tolerated, with adverse events being mild and self-limited.
Conclusion: In this first prospective controlled study, RZV demonstrated favorable safety and high rates of immunogenicity in PsA and axSpA patients under immunosuppressive treatment. However, the lower frequency of seroconversion and reduced antibody titers compared to CG raise concerns about vaccine-induced protection. These findings support the use of RZV in high-risk patients and highlights the need for long-term monitoring of vaccine efficacy. (ClinicalTrials NCT05879419).
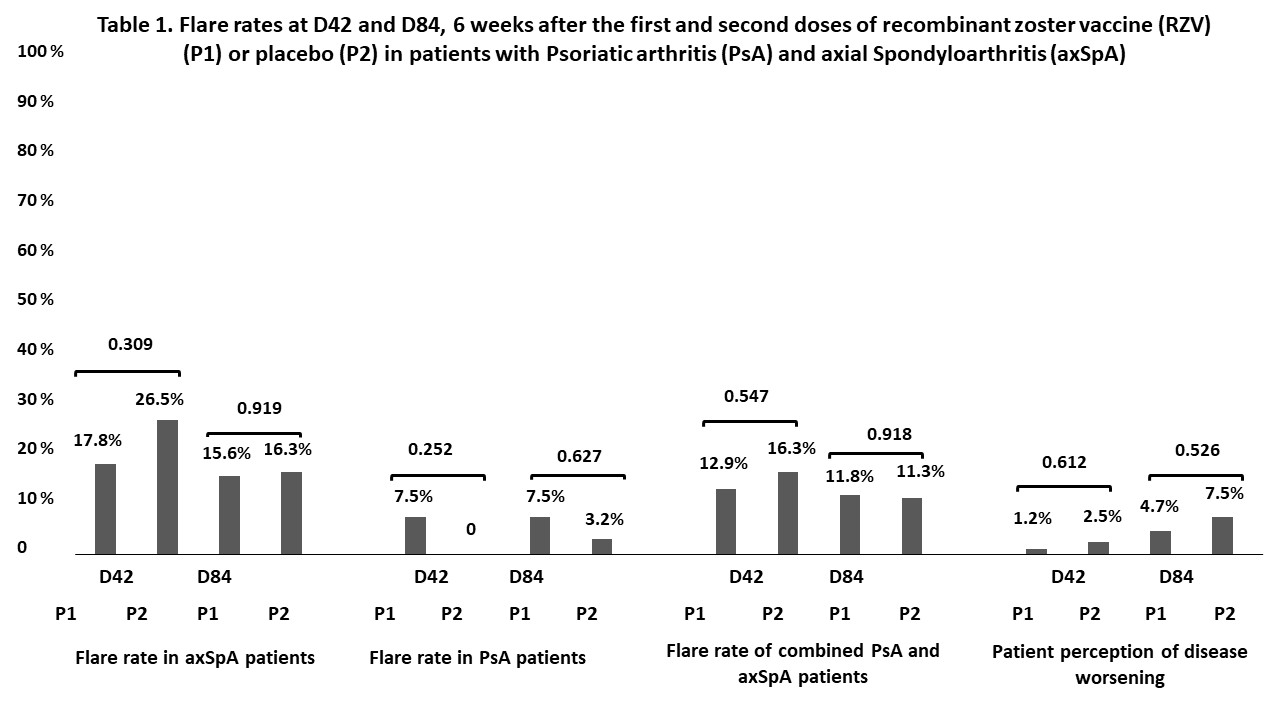 For safety analyses, all patients who adhered to protocol were included (n=165): 71 axSpA patients (40 in P1 and 31 in P2) and 94 PsA patients (45 in P1 and 49 in P2). Results are expressed in n (%) and compared with the chi-square or Fisher’s exact test, as appropriate, as two-sided analyses; Flare rate in axSpA patients was defined as an increase > 0.9 in ASDAS-CRP. Flare rate in PsA patients was defined as worsening in DAPSA category. ASDAS-CRP, DAPSA classification and patient perception of disease worsening at D42 and D84 were compared to baseline status (D0)
For safety analyses, all patients who adhered to protocol were included (n=165): 71 axSpA patients (40 in P1 and 31 in P2) and 94 PsA patients (45 in P1 and 49 in P2). Results are expressed in n (%) and compared with the chi-square or Fisher’s exact test, as appropriate, as two-sided analyses; Flare rate in axSpA patients was defined as an increase > 0.9 in ASDAS-CRP. Flare rate in PsA patients was defined as worsening in DAPSA category. ASDAS-CRP, DAPSA classification and patient perception of disease worsening at D42 and D84 were compared to baseline status (D0)
To cite this abstract in AMA style:
Saad C, Borba E, Chaer F, Medeiros-Ribeiro A, Pasoto S, Aikawa N, Correia L, Sampaio-Barros P, Moraes J, Dorio M, Goldenstein-Schainberg C, Silva C, Bonfa E. Spondyloarthritis and Psoriatic Arthritis Patients Under Immunosuppressive Conventional and Biologic DMARDs: Favorable Disease Safety and Attenuated Immunogenicity Following Recombinant Herpes Zoster Vaccination [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/spondyloarthritis-and-psoriatic-arthritis-patients-under-immunosuppressive-conventional-and-biologic-dmards-favorable-disease-safety-and-attenuated-immunogenicity-following-recombinant-herpes-zoster/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/spondyloarthritis-and-psoriatic-arthritis-patients-under-immunosuppressive-conventional-and-biologic-dmards-favorable-disease-safety-and-attenuated-immunogenicity-following-recombinant-herpes-zoster/
