Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Spirulina, a popular herbal supplement, stimulates the immune system, as determined by in vitro and in vivo studies. Our recent epidemiologic data suggest that Spirulina is associated with the onset or exacerbation of pre-existing autoimmune skin diseases, such as DM. The purpose of this study was to investigate the immunostimulatory effects of Spirulina and characterize the role of the Stimulator of interferon genes (STING) and Toll-like Receptor (TLR) pathways.
Methods: PBMCs were isolated from DM and normal controls and stimulated with increasing concentrations of pure Spirulina supernatant (0, 0.3, and 1 mg/ml). All DM patients met the EULAR/ACR definition of DM or amyopathic DM. PBMCs were also pre-treated for 1 hour with the following inhibitors: STING inhibitor, H-151; TLR4 inhibitor, TAK 242; TLR2 and TLR4 inhibitor, Sparstolonin B; and TBK1 inhibitor, MRT67307. Cells were incubated for 18 hours before supernatant was collected for ELISA.
Results: Spirulina dose-dependently increases PBMC production of IFNβ in DM patients (Figure 1a). Spirulina also increases PBMC production of TNFα, with peak levels when stimulated with 0.3 mg/ml Spirulina (Figure 1b).
In the presence of Spirulina, STING inhibitor suppressed secretion of IFNβ in all five DM patients. “Good Responders,” defined as subjects with >20% decrease in cytokine levels with STING inhibitor and stimulation at both Spirulina concentrations had mean (standard error of mean) IFNβ levels of 33.78 (1.51) pg/ml pre-antagonist and 13.19 (7.11) post-antagonist when stimulated with Spirulina 0.3 mg/ml and IFNβ levels of 59.47 (7.04) pg/ml pre-antagonist and 33.81 (8.17) post-antagonist when stimulated with Spirulina 1 mg/ml (p=0.06) (Figure 2).
With Spirulina stimulation, STING inhibitor only suppressed secretion of TNFα in 6/15 subjects and these results were not statistically significant. A second experiment using 3 subjects further examined the effects of TLR2 and TLR4, TLR2 and TLR4 and STING, TBK1, or TLR4 inhibition on PBMC production of TNFα. In the presence of 0.3 mg/ml Spirulina stimulation, these four inhibitors significantly decreased TNFα levels (Figure 3) with TNFα decreasing from mean (standard error of mean) 1829 (243.47) pg/ml to 384.6 (149.27), 346.0 (138.11) 410.7 (73.43), and 175.7 (17.68) pg/ml when TLR2 and TLR4, TLR2 and TLR4 and STING, TBK1, or TLR4 inhibitors were added, respectively (n=3) (p< 0.0001). STING inhibition decreased TNFα production from 1829 pg/ml to 1488 pg/ml (n=3) (p=0.84).
Conclusion: Our preliminary results show that Spirulina increases production of key inflammatory cytokines TNFα and IFNβ. For TNFα production, Spirulina’s immunostimulatory effects appear to be primarily mediated via the TLR4 pathway. Inhibition of TBK1, an important kinase in the innate immune system active in both the STING and NF-kappa B pathways, also significantly decreases TNFα production. For IFNβ production, Spirulina’s immunostimulatory effects appear to be partially mediated by the STING pathway. Our findings provide a potential mechanism by which Spirulina use may lead to disease onset or flare in susceptible patients.
 Figure 1. (a) DM PBMCs secreted mean (standard error of mean) IFNβ of 14.18 (4.18), 38.26 (4.69), and 97.42 (23.76) pg/ml, at 0, 0.3, and 1 mg/ml Spirulina concentrations, respectively (n=6). (b) DM PBMCs secreted mean (standard error of mean) TNFα of 213.03 (101.08), 1718.94 (208.95), and 938.36 (134.72) pg/ml at 0, 0.3, and 1 mg/ml Spirulina concentrations, respectively (n=17). Bars show * p < 0.05, ** p < 0.01, **** p < 0.0001. Line represents the median.
Figure 1. (a) DM PBMCs secreted mean (standard error of mean) IFNβ of 14.18 (4.18), 38.26 (4.69), and 97.42 (23.76) pg/ml, at 0, 0.3, and 1 mg/ml Spirulina concentrations, respectively (n=6). (b) DM PBMCs secreted mean (standard error of mean) TNFα of 213.03 (101.08), 1718.94 (208.95), and 938.36 (134.72) pg/ml at 0, 0.3, and 1 mg/ml Spirulina concentrations, respectively (n=17). Bars show * p < 0.05, ** p < 0.01, **** p < 0.0001. Line represents the median.
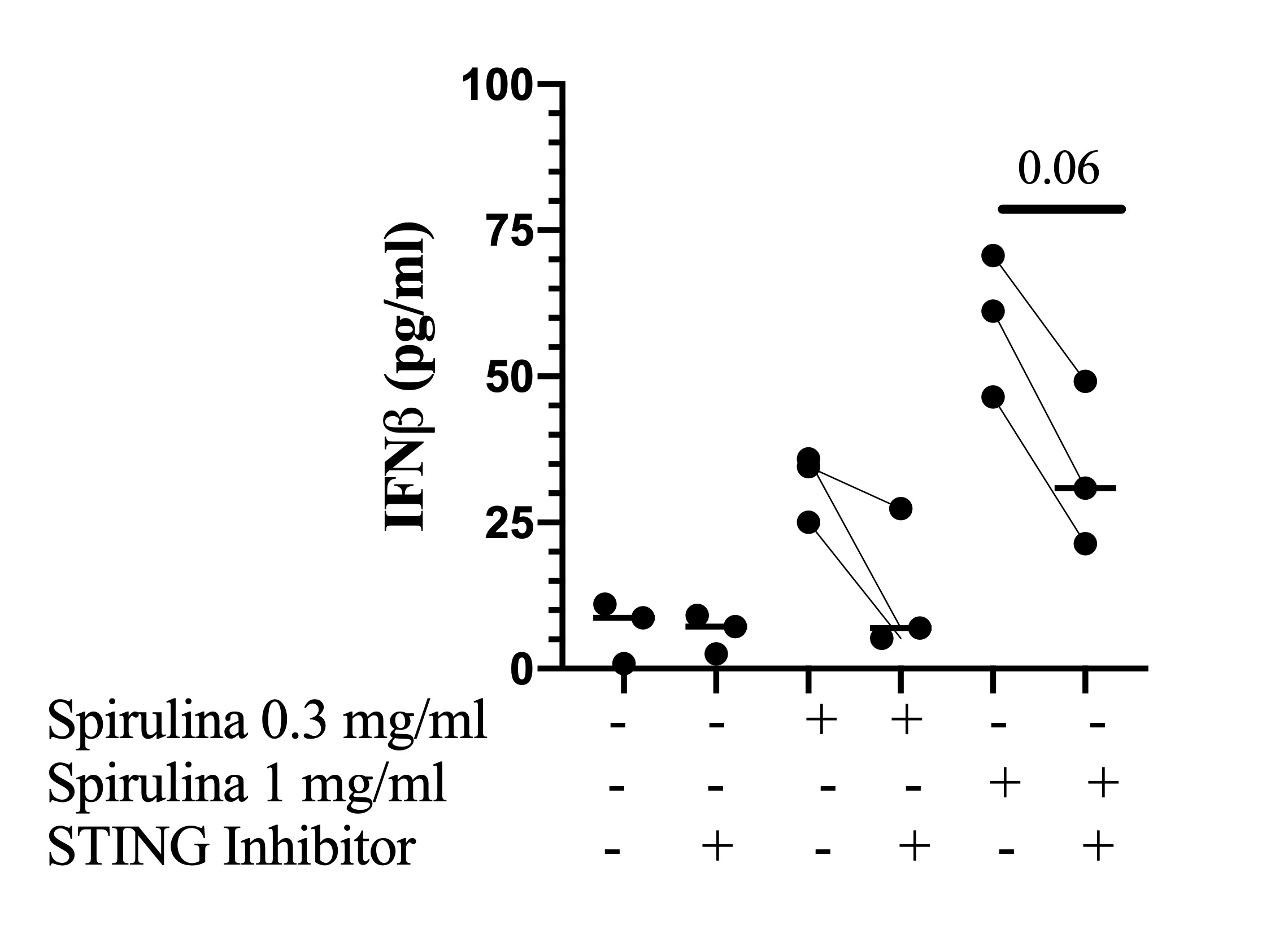 Figure 2. Effects of STING inhibitor versus sham on secretion of IFNβ from unstimulated and Spirulina-stimulated PBMCs in “Good Responders.” Line represents the median. (n=3)
Figure 2. Effects of STING inhibitor versus sham on secretion of IFNβ from unstimulated and Spirulina-stimulated PBMCs in “Good Responders.” Line represents the median. (n=3)
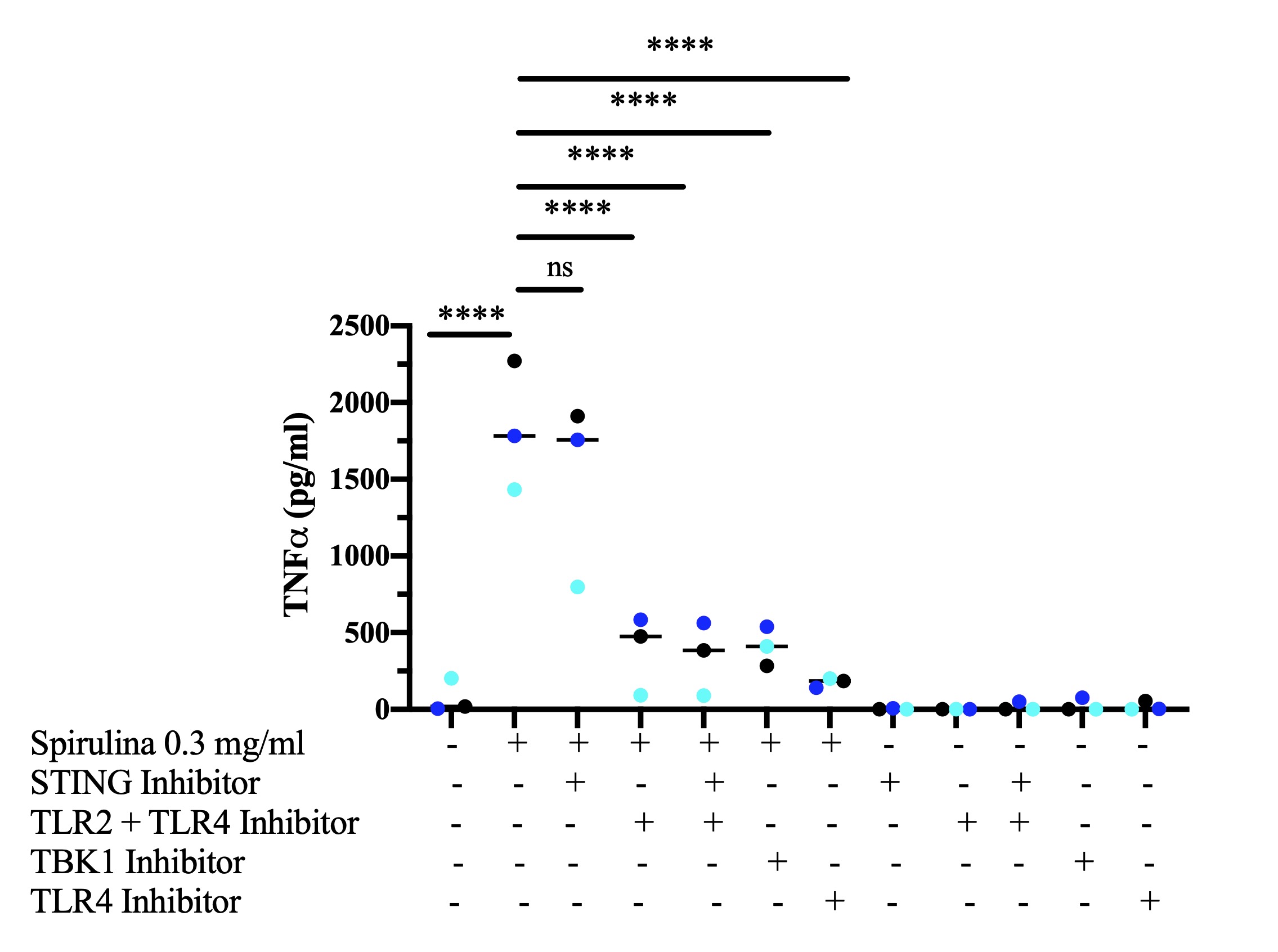 Figure 3. Effects of STING, TLR2 + TLR4, TLR2 + TLR4 + STING, TBK1, or TLR4 inhibition versus sham on secretion of TNFα from unstimulated and Spirulina-stimulated PBMCs (n=3). Bars show **** p < 0.0001. Line represents the median.
Figure 3. Effects of STING, TLR2 + TLR4, TLR2 + TLR4 + STING, TBK1, or TLR4 inhibition versus sham on secretion of TNFα from unstimulated and Spirulina-stimulated PBMCs (n=3). Bars show **** p < 0.0001. Line represents the median.
To cite this abstract in AMA style:
Bax C, Li Y, Maddukuri S, Ravishankar A, Patel J, Yan D, Concha J, Werth V. Spirulina Stimulates Inflammatory Cytokine Production Through the STING and TLR Pathways in Dermatomyositis in Vitro [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/spirulina-stimulates-inflammatory-cytokine-production-through-the-sting-and-tlr-pathways-in-dermatomyositis-in-vitro/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/spirulina-stimulates-inflammatory-cytokine-production-through-the-sting-and-tlr-pathways-in-dermatomyositis-in-vitro/
