Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Hyperuricemia (HU) is reported as a risk factor for gout and associates with diverse diseases, including ulcerative colitis (UC), while its mechanism remains unclear. In this study, we aim to explain UA effect based on the hypothesis that UA-binding proteins function as UA sensors and regulate various physiological processes.
Methods: UA-binding proteins were detected by proteomics following a pull-down assay with UA-binding beads and human colorectal Caco-2 cells homogenate. Specific binding of these candidate binding proteins was confirmed by binding assay with in vitro synthesized proteins using cell-free method. UA was exposed to the cells after filtering by 0.2 μm filter to avoid UA crystals. The cellular levels of iron, ROS, and lipid peroxidation (MDA) were measured with FerroOrange, CellROX®, MDA assay kit, respectively.
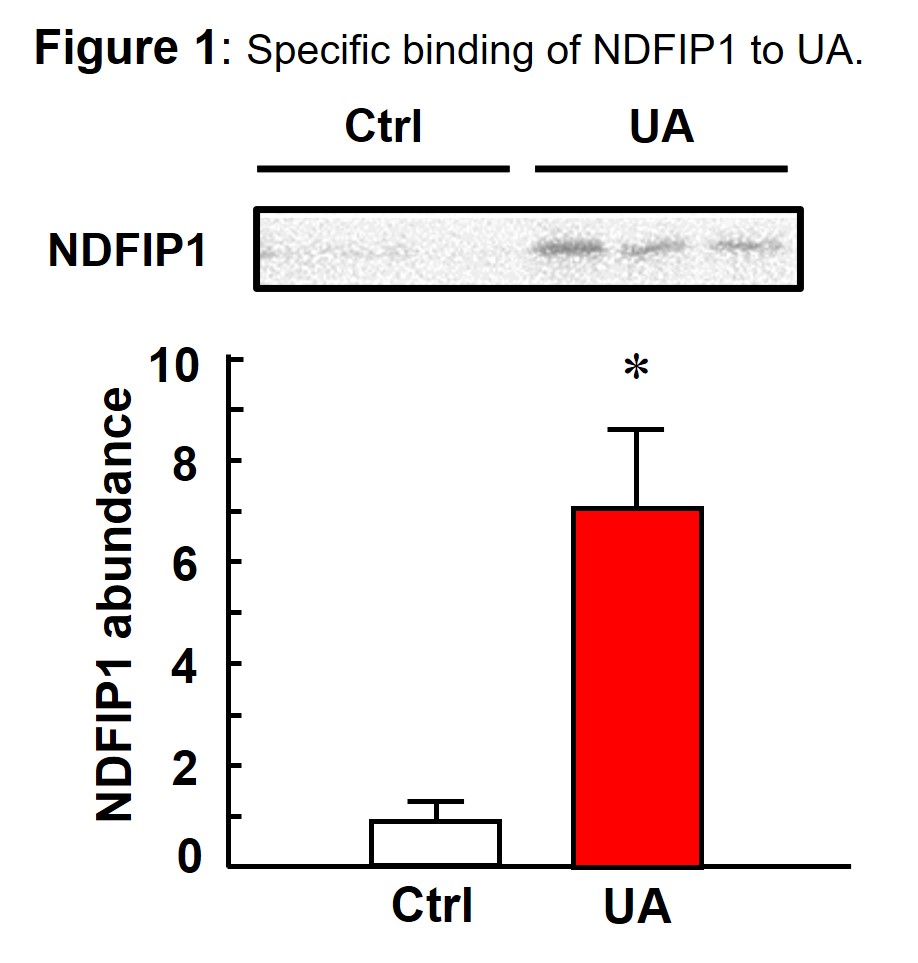
Results: Six proteins were found to be potential to bind to UA by pull-down assay. Specific binding of UA to these proteins was confirmed by binding assay using in vitro synthesized proteins, where NDFIP1 showed the highest binding rate (Fig. 1). Binding domain was narrowed down by binding assay with UA-binding beads and in vitro synthesized partial NDFIP1 protein. To find the binding sites, docking simulation of AlphaFold2-predicted NDFIP1 and UA was carried out. As a result, intracellular C-terminal sequences of NDFIP1 was suggested as UA-binding region.
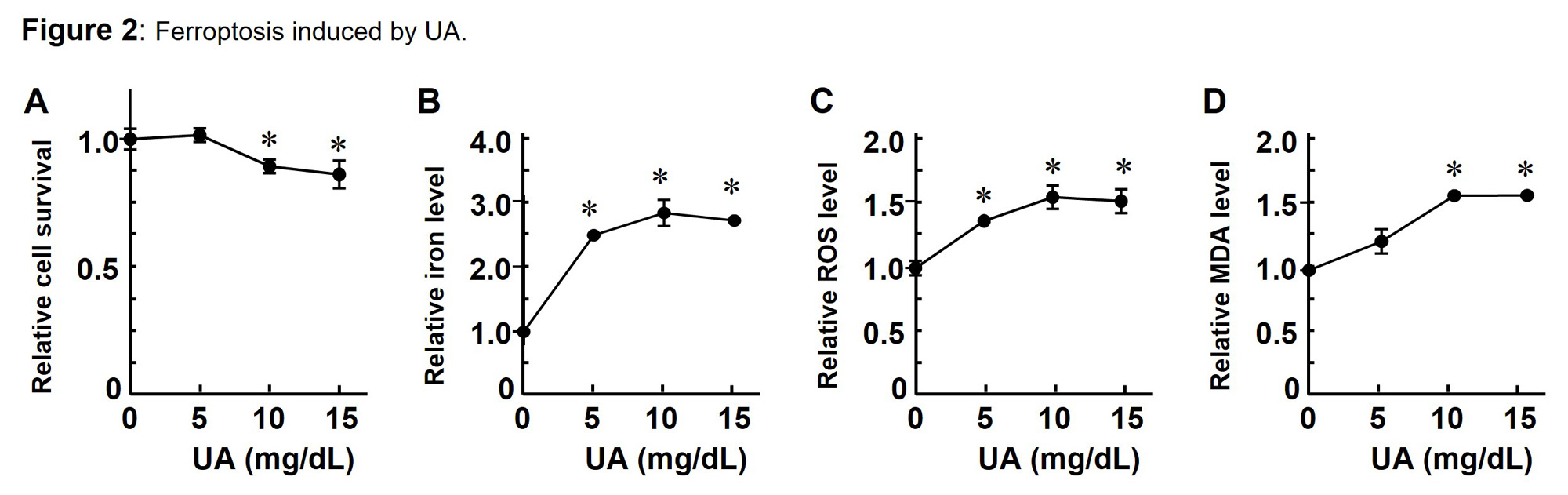
Exposing UA to Caco-2 cells caused cell death and the viability decreased with increasing UA concentration (Fig. 2A). In addition, as UA significantly increased intracellular iron, ROS and MDA level (Fig. 2B-D), suggesting that high UA level could induce ferroptosis, which is associated with UC.
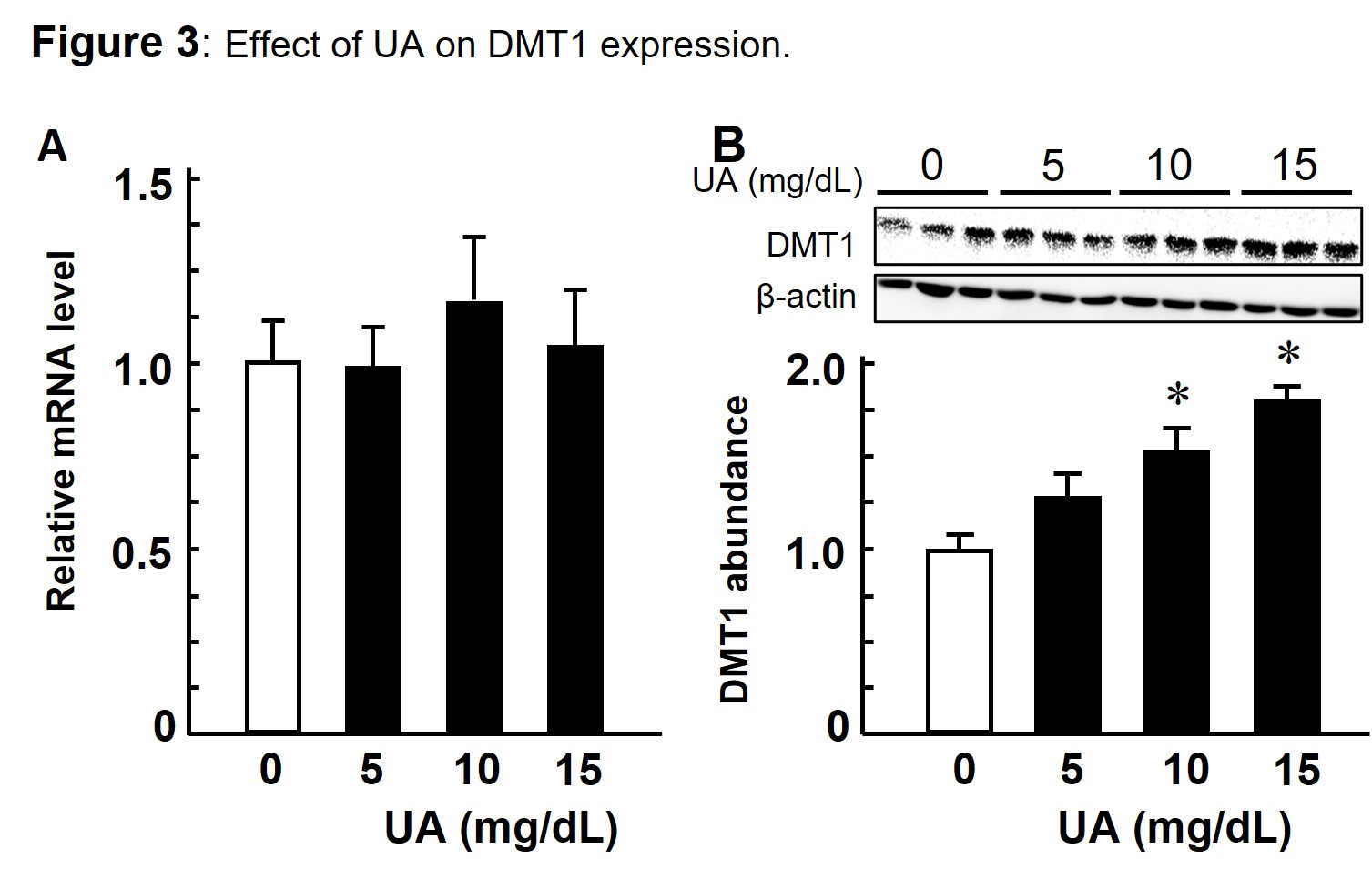
Since NDFIP1 is known as a ubiquitin ligase adaptor and promotes ubiquitination of DMT1 (divalent metal transporter 1, an iron transporter), mRNA and protein expressions of DMT1 were measured as Fig. 3, where UA increased DMT1 protein significantly but not mRNA level.
Exposure of DMT1 blocker 2 reduced UA-induced ferroptosis. Furthermore, knockdown of NDFIP1 by transfecting siRNA lowered the difference of ferroptosis level between UA exposed and control groups.
Conclusion: NDFIP1 was found as UA binding protein and was suggested as a UA sensor that regulates HU-induced ferroptosis by modulating DMT1.
NDFIP1 bound to UA-binding beads was approximately 7 times as much as that of control.
Exposing UA to Caco_2 cells caused cell death (A) and led to higher intracellular iron (B), ROS (C), and MDA (D) levels.
Exposing UA to Caco_2 cells increased DMT1 protein expression significantly (B), but not the mRNA level (A).
To cite this abstract in AMA style:
Zhu Q, Arakawa H, Shirasaka Y, Tamai I. Specific Binding of Uric Acid to NDFIP1 Associates with Hyperuricemia-induced Ferroptosis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/specific-binding-of-uric-acid-to-ndfip1-associates-with-hyperuricemia-induced-ferroptosis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/specific-binding-of-uric-acid-to-ndfip1-associates-with-hyperuricemia-induced-ferroptosis/