Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Spondyloarthritis (SpA) significantly affects patients’ quality of life (QoL). Current tools in routine medical practice primarily focus on assessing disease activity, neglecting the broader impact of SpA on various facets of QoL. To address this critical gap, our objective was to develop the SpA Disk©, a straightforward yet comprehensive tool. This instrument aims to evaluate the burden of SpA on essential aspects of daily life often overlooked during physician-patient consultations.
Methods: The SpA Disk© was developed through a standardized process. Specific domains of interest were selected based on a literature review of existing Patient-Reported Outcomes (PROs) and QoL questionnaires. Input from SpA patients, encompassing axial SpA, psoriatic arthritis, and SAPHO syndrome, was collected through two online surveys. A face-to-face meeting with SpA experts was convened to craft the initial version of the disk. An online validation process involving SpA experts was conducted to validate the preliminary questionnaire, resulting in a fully validated SpA Disk©.
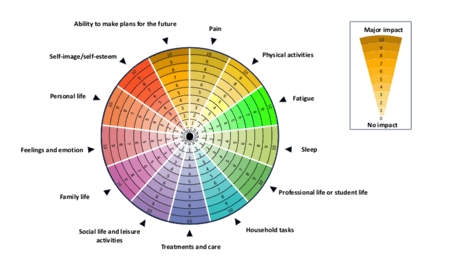
Results: The finalized SpA Disk© comprises 13 items presented in the format of a numerical scale shaped like a disk. This scale ranges from 0 (signifying absolute disagreement/no impact) to 10 (indicating complete agreement/major impact) and covers a 30-day time period. Each item on the disk is supplemented with explanatory statements and keywords, offering context for respondents. These accompanying details serve as a guide for application, enhancing the questionnaire’s usability and understanding.
Conclusion: The SpA Disk© emerges as a user-friendly, concise, and all-encompassing self-reporting tool, addressing every crucial aspect of SpA’s potential impact on patients’ QoL. Through its visual representation, the SpA Disk© provides a clear depiction of this impact, aiding physicians not only during patient visits but also in outpatient settings.
To cite this abstract in AMA style:
Ghossan R, FOGEL O, Gaujoux-Viala C, SABER a, Lukas C, MICELI C. SpA Disk©: A New Tool to Monitor the QoL of Spondyloarthritis Patients [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/spa-disk-a-new-tool-to-monitor-the-qol-of-spondyloarthritis-patients/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/spa-disk-a-new-tool-to-monitor-the-qol-of-spondyloarthritis-patients/