Session Information
Date: Monday, November 13, 2023
Title: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes II: Omics
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: The G1 and G2 risk variants (RVs) in Apolipoprotein L1 (APOL1) associate with CKD and may contribute to poorer outcomes for African American (AA) patients with lupus nephritis (LN). While the pathogenetic mechanism for APOL1 related CKD remains unknown, most studies focus on glomerular injury. This study leveraged the multi-center LN AMP to evaluate APOL1 RV associated clinical phenotypes and identify whether these genetic variants influence the transcriptomic landscape in kidney cells.
Methods: LN patients were consecutively enrolled in AMP at the time of a clinically indicated renal biopsy and followed for one year. Dissociated biopsies were passed through a droplet-based single-cell RNA sequencing (scRNAseq) pipeline that included quality control of sequenced libraries. Genotypes for APOL1 RVs were identified by sanger sequencing for all AA patients enrolled with available DNA.
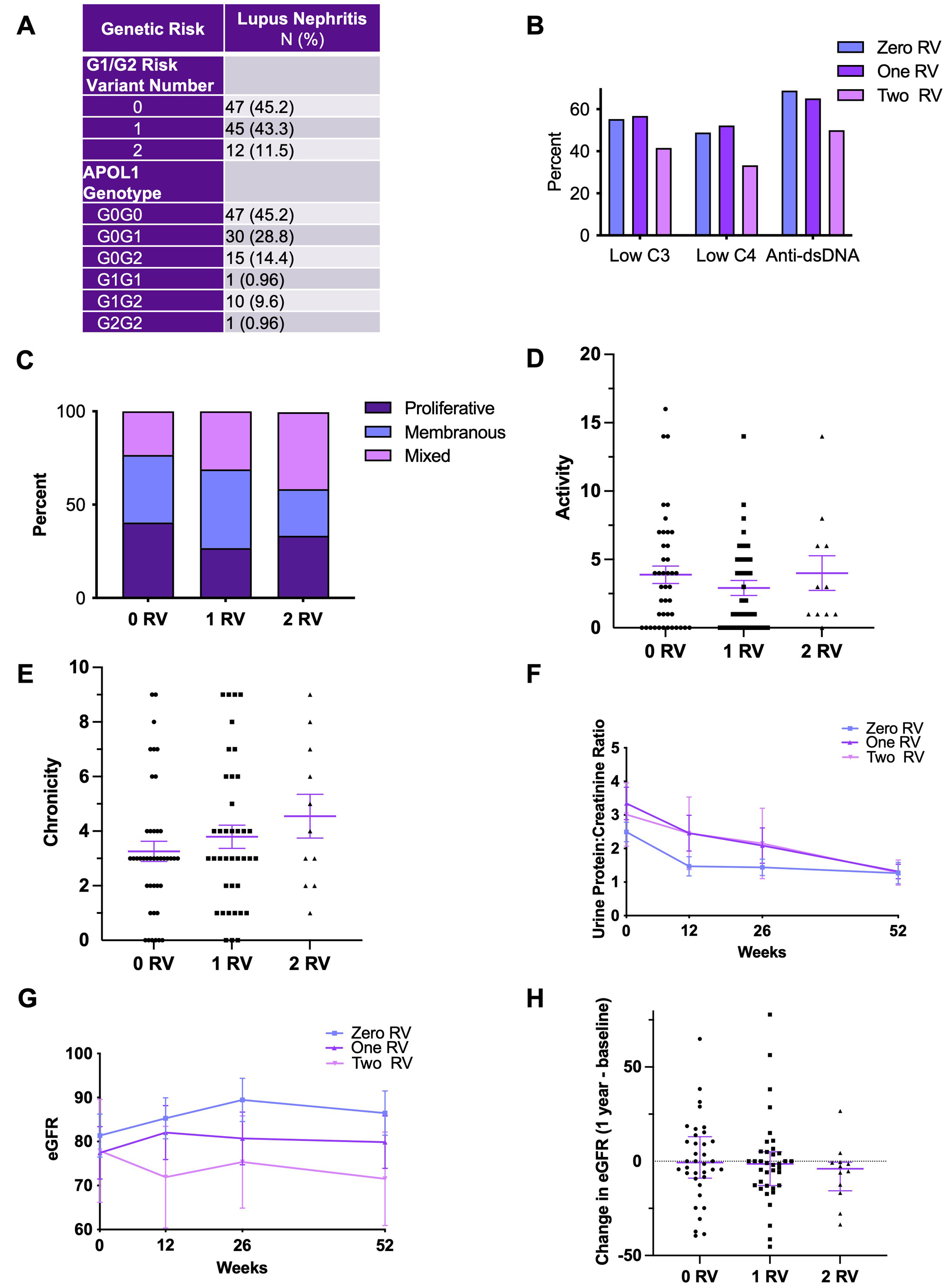
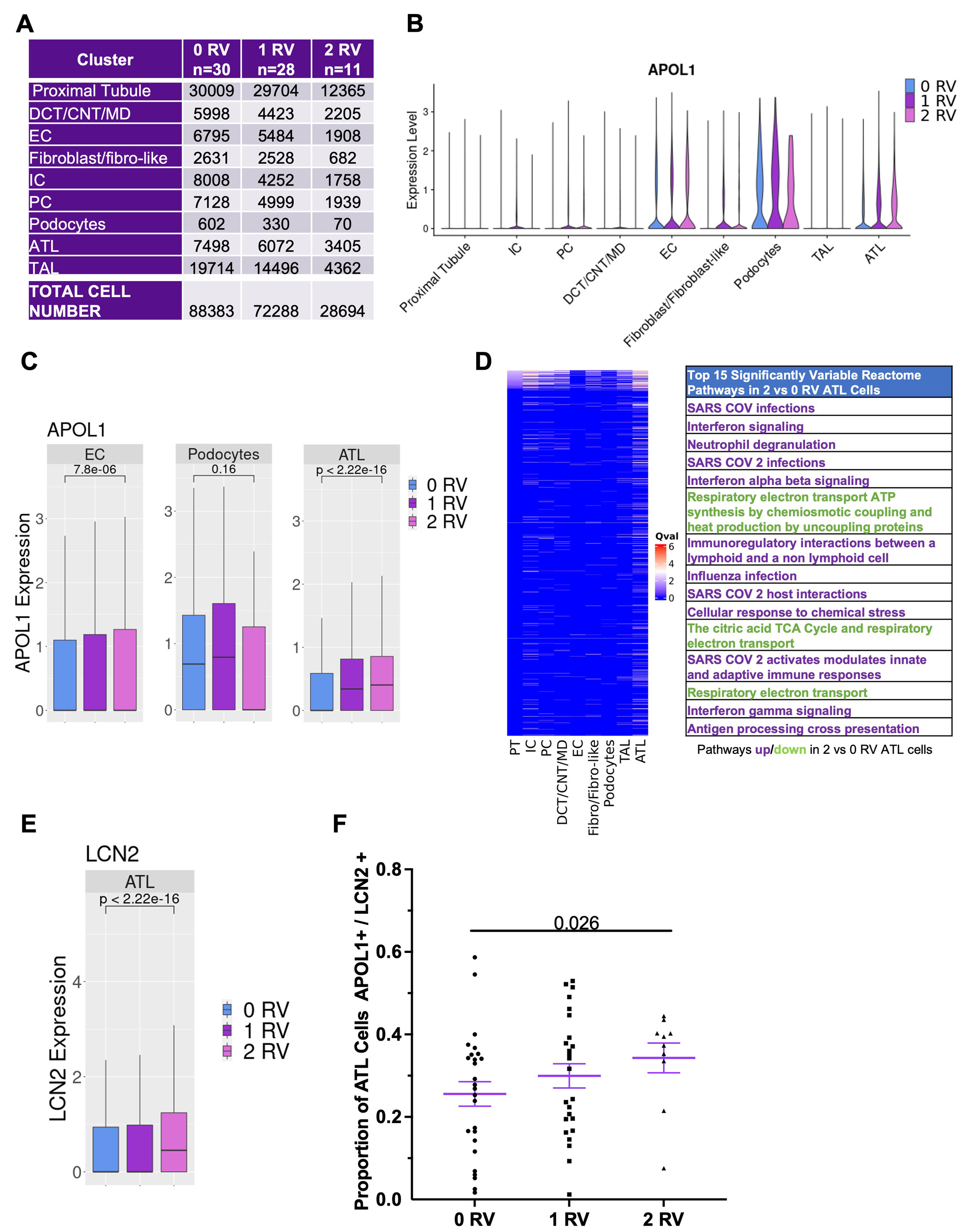
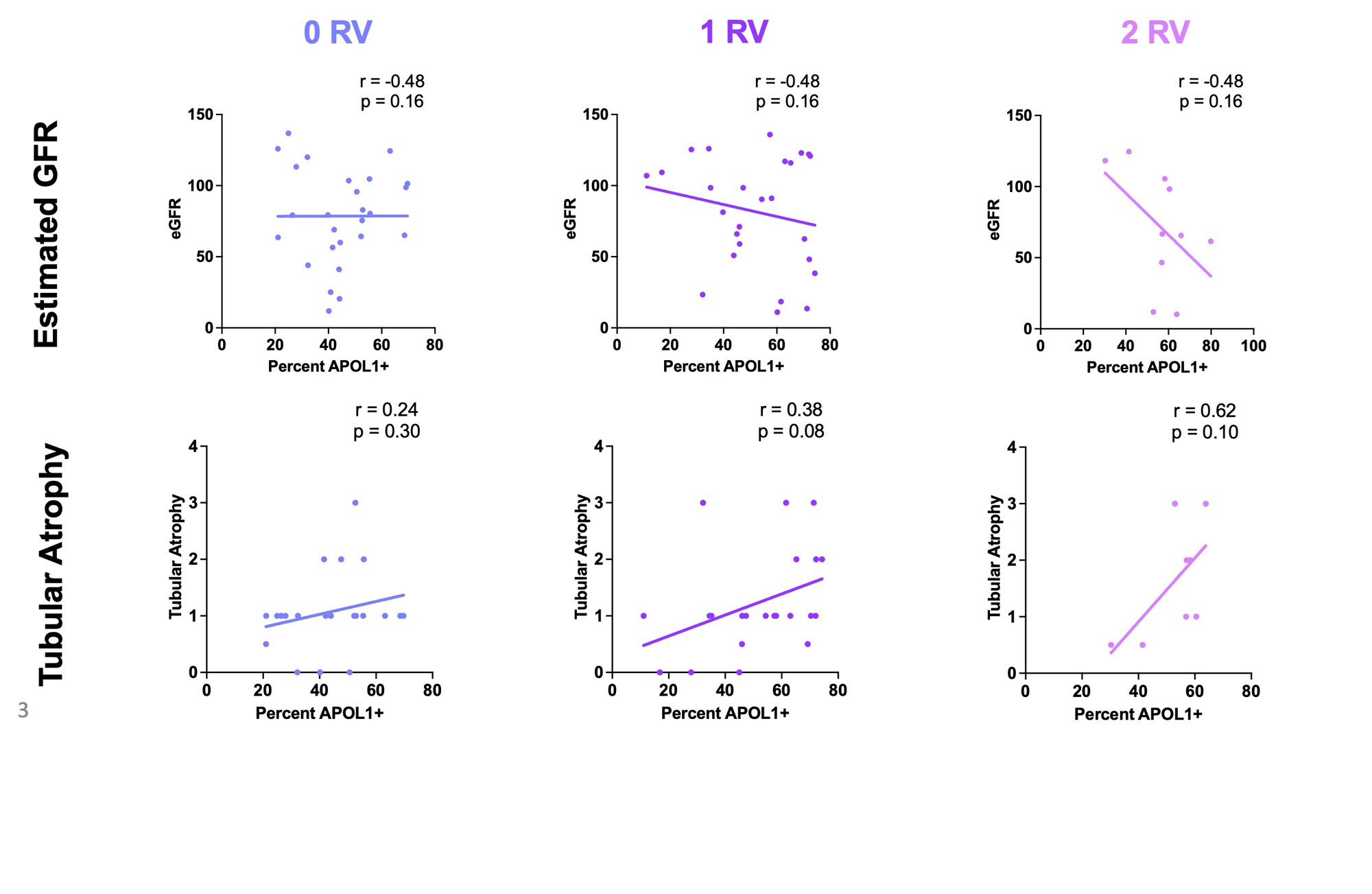
Results: In total, 104 AA patients were genotyped; 47 (45.2%) carried zero APOL1 RVs, 45 (43.3%) one RV, and 12 (11.5%) two RVs. RVs did not associate with baseline anti-dsDNA or complement levels, biopsy class/activity/chronicity, GFR or proteinuria (Fig. 1A-G). While there was a trend toward decreased GFR at one year by gene variant dosage, there was no association with changes in proteinuria (Fig. 1F-H). ScRNAseq yielded 88383 high quality cells in patients with zero RVs (n=30), 72288 one RV (n=28), and 28694 two RVs (n=11) spanning nine parenchymal cluster types (Fig. 2A). Independent of genotype, APOL1 expression was highest in podocyte, endothelial and ascending thin limb (ATL) cells (Fig. 2B). Median APOL1 expression was significantly higher in cells with one or two RVs in the ATL cluster but this association was not seen in podocytes or endothelial cells (Fig. 2C). Single cell pathway analysis revealed that the ATL cluster demonstrated greater pathway level variation between cells with two RVs vs zero than any other cluster, with the most distinguishing related to interferon signaling (increased), antigen presentation (increased), and mitochondrial function (decreased) (Fig. 2D). The ATL damage associated gene, lipocalin-2 (LCN2), was expressed at significantly higher levels in cells carrying two RVs (Fig. 2E).Likewise, the proportion of ATL cells double positive for APOL1 and LCN2 was higher in patients with two RVs (Fig. 2F). While it did not reach significance, the percentage of ATL cells expressing APOL1 more strongly correlated with eGFR and tubular atrophy on biopsy histology among patients with two RVs compared to those carrying zero or one RV (Fig. 3A-F).
Conclusion: APOL1 RVs associated with decreased GFR but not proteinuria suggesting that current clinical indicators of LN severity may not appropriately prognosticate patients carrying APOL1 RVs and that the use of routine genotyping in the clinical setting may better risk stratify AA patients with LN. The scRNAseq data revealed that ATL cells likely express APOL1 and that this may be relevant to progressive kidney dysfunction over time. This highlights the potential for a previously unrecognized extraglomerular injury in AA SLE patients carrying APOL1 RVs providing a novel future direction for understanding APOL1 toxicity and translation to clinical trials.
To cite this abstract in AMA style:
Carlucci P, Shwetar J, Gurajala S, Xiao Q, Mears J, Preisinger K, Zaminski D, Deonaraine K, Izmirly P, Fava A, James J, Guthridge J, Rovin B, Madhavan S, DeJager W, Wofsy D, Wu M, Putterman C, Rao D, Diamond B, Fine D, Monroy-Trujillo J, Haag K, Belmont H, Apruzzese W, Davidson A, Payan-Schober F, Furie R, Hoover P, Berthier C, Dall'Era M, Cho K, Kamen D, Kalunian K, Anolik J, Arazi A, Raychaudhuri S, Hacohen N, Petri M, Clancy R, Ruggles K, Buyon J, in RA/SLE T. Single Cell Transcriptomics in Kidney Tissue from African American Patients Enrolled in the Accelerating Medicines Partnership (AMP) Implicates Tubular Cells in the Pathogenesis of APOL1 Associated Lupus Nephritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/single-cell-transcriptomics-in-kidney-tissue-from-african-american-patients-enrolled-in-the-accelerating-medicines-partnership-amp-implicates-tubular-cells-in-the-pathogenesis-of-apol1-associated-lu/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/single-cell-transcriptomics-in-kidney-tissue-from-african-american-patients-enrolled-in-the-accelerating-medicines-partnership-amp-implicates-tubular-cells-in-the-pathogenesis-of-apol1-associated-lu/