Session Information
The 2020 Pediatric Rheumatology Symposium, originally scheduled for April 29 – May 2, was postponed due to COVID-19; therefore, abstracts were not presented as scheduled.
Date: Friday, May 1, 2020
Title: Plenary Abstracts Session 2
Session Type: Plenary Session II
Session Time: 2:30PM-3:00PM
Background/Purpose: Scleroderma is an autoimmune disorder involving inflammatory driven fibrosis, which encompasses systemic sclerosis (SSc) and localized scleroderma (LS). LS and SSc share histological characteristics, inflammatory and fibrotic processes, but likely have unique pathways defining the interaction between inflammation and fibrosis given divergent clinical phenotypes. Recent research indicates that T cells, macrophages, and related cytokines interact with fibroblasts to initiate an inflammatory phase followed by fibrosis. Identifying inflammatory cells expressing IFNγ-associated genes of interest, identified in our recent RNA bulk sequencing skin work, and unique fibroblast populations will allow mechanistic studies of inflammatory-driven LS fibrosis, leading to more effective therapies.
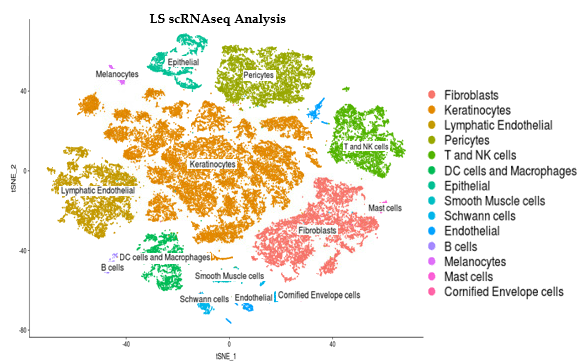
Methods: Single cell RNA sequencing (scRNAseq) was performed on the skin of 11 LS patients (5 pediatric & 6 adult) and 10 healthy age-matched controls. Library preparation was done on enzymatically digested cells using a 10X Genomics® Chromium instrument and sequencing was performed on Illumina NextSeq or HighSeq instruments. ScRNA-seq reads were examined for quality then transcripts were mapped to reference human genome GRCh38 and assigned to cells of origin using the Cell Ranger pipeline (10X Genomics®). R-language analyses using Seurat identified and visualized distinct cell populations by clustering methodologies.
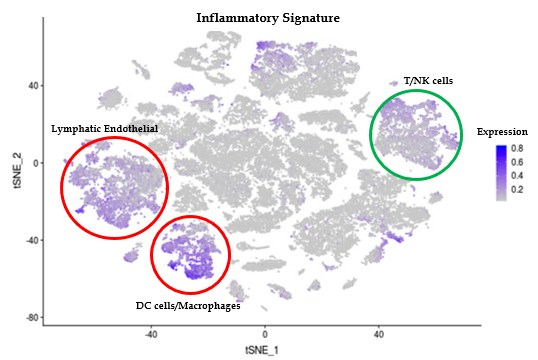
Results: LS and healthy skin cells clustered into 28 unique cell population groups. Earlier bulk RNA sequencing of LS compared to control samples found and inflammatory gene signature related to IFNγ and TNFα pathways, leukocyte activation and/or regulation, and cytokine production. These inflammatory genes in LS scRNAseq data were highly expressed in 3 main cell clusters: endothelial, lymphocyte/NK, and macrophage/DC. Sub-clustering showed high IFNγ signaling with CXCR3 and related chemokines (CXCL9 and CXCL10) being expressed from T cell and M1/M2 macrophages. Since chemokine expression from these inflammatory cells might stimulate fibroblasts and affect the degree of LS fibrosis, fibroblast populations were also studied. LS cells formed unique fibroblast clusters defined by COL1A1, SFRP2, and CXCL12 that expressed both inflammatory genes, like CXCL9 and 10, and fibrotic genes, like IGFBP5. Some of these genes expressed on LS fibroblasts are shared with recent findings in SSc scRNAseq, however the more inflammatory gene signature expressed on LS fibroblasts may be unique.
Conclusion: IFNy associated gene transcripts including CXCR3 ligands (CXCL9 and CXCL10) are prevalent in macrophage, lymphocyte, and fibroblast populations in LS skin. The unique fibroblast subsets expressing these CXCR3 ligands, only found in LS skin, co-express reticular dermis fibroblast markers. Progression of inflammatory expression of these populations will be further investigated using advanced analysis techniques to determine the cellular trajectory and interaction of LS cells.
To cite this abstract in AMA style:
Mirizio E, Chen W, Sun T, Tabib T, Schollaert-Fitch K, Lafyatis R, Jacobe H, Torok K. Single Cell Sequencing of the Skin to Define Cell Populations of Interest in Localized Scleroderma (LS) [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 4). https://acrabstracts.org/abstract/single-cell-sequencing-of-the-skin-to-define-cell-populations-of-interest-in-localized-scleroderma-ls/. Accessed .« Back to 2020 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/single-cell-sequencing-of-the-skin-to-define-cell-populations-of-interest-in-localized-scleroderma-ls/