Session Information
Date: Monday, October 27, 2025
Title: Plenary II (0849–0854)
Session Type: Plenary Session
Session Time: 8:15AM-8:30AM
Background/Purpose: CD4+ T cells play a central role in the pathogenesis of human rheumatoid arthritis (RA). However, therapies targeting CD4+ T cell-derived humoral factors, including IL-17, have shown limited clinical efficacy. This suggests that the key CD4⁺ T cell subsets and associated effector molecules driving synovial inflammation in RA remain incompletely understood. To address this, we performed a comprehensive single-cell transcriptomic analysis of synovial CD4⁺ T cells from RA patients.
Methods: Single-cell RNA sequencing (scRNA-seq) was performed on CD4+ T cells in the synovial tissue and synovial fluid of 11 seropositive RA patients (all female, mean age 65.6 years, mean DAS28-ESR 4.21). The function of an inflammatory factor identified in the analysis was assessed through in vitro experiments including CRISPR-Cas9. Serum levels of this factor were investigated in 30 control participants and 30 RA patients.
Results: scRNA-seq identified CD4+ T cell clusters, including PD-1+CXCL13+ peripheral helper T (Tph), regulatory T (Treg), cytotoxic T, and Th17 cells. The frequency of Tph cells showed a significant correlation with disease activity, whereas the frequency of Treg and Th17 cells did not. We further identified a humoral factor, pathogenic helper inflammatory factor (PHIF, tentative gene name), with whose expression correlated with disease severity. PHIF was specifically expressed by human CD4+ T cells, predominantly within Tph cells. In vitro experiments showed that PHIF promoted CXCL13 production in Tph cells. In addition, PHIF activated NF-κB signaling in monocytes and induced gene signatures associated with pathogenic macrophages from RA synovial tissue, including CXCL10 and CXCL9. Consistently, CRISPR/Cas9-knockout of PHIF in patient-derived synovial Tph cells suppressed the activation of CXCL10 and IFN signature in co-cultured monocytes (Figure 1). Notably, serum PHIF protein levels significantly correlated with RA disease severity and effectively discriminated RA patients, comparable to established biomarkers such as anti-citrullinated antibodies (Figure 2).
Conclusion: These findings highlight the substantial involvement of PHIF in RA pathogenesis, emphasizing how human Tph cells exert pleiotropic regulation of local immune responses via PHIF in addition to promoting antibody production via CXCL13. Thus, PHIF can be a potential biomarker and therapeutic target for RA.
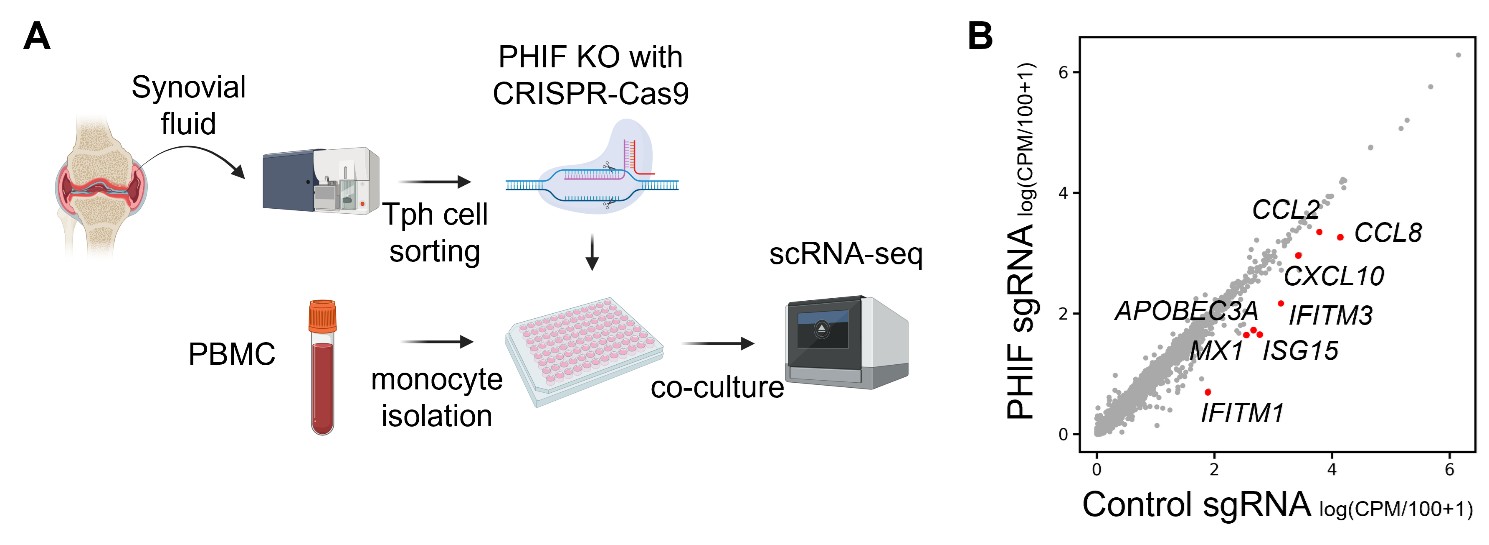 Figure 1. Effects of PHIF knockout in Tph cells on monocyte gene expression. (A) Tph cells sorted from synovial CD4+ T cells were treated with PHIF knockout using CRISPR-Cas9 and were co-cultured with PBMC monocytes, followed by scRNA-seq analysis. (B) A scatter plot comparing RNA expression of control sgRNA and PHIF sgRNA in co-cultured monocytes.
Figure 1. Effects of PHIF knockout in Tph cells on monocyte gene expression. (A) Tph cells sorted from synovial CD4+ T cells were treated with PHIF knockout using CRISPR-Cas9 and were co-cultured with PBMC monocytes, followed by scRNA-seq analysis. (B) A scatter plot comparing RNA expression of control sgRNA and PHIF sgRNA in co-cultured monocytes.
.jpg) Figure 2. Clinical assessment of PHIF in RA. (A) Blood PHIF protein levels between control (n = 30) and RA (n = 30). (B) ROC curve of blood PHIF levels for RA determination with the AUC value. The means ± SD are shown in (A). ****p < 0.0001; Mann-Whitney U tests (A). ROC, receiver operating characteristic; AUC, area under the curve.
Figure 2. Clinical assessment of PHIF in RA. (A) Blood PHIF protein levels between control (n = 30) and RA (n = 30). (B) ROC curve of blood PHIF levels for RA determination with the AUC value. The means ± SD are shown in (A). ****p < 0.0001; Mann-Whitney U tests (A). ROC, receiver operating characteristic; AUC, area under the curve.
To cite this abstract in AMA style:
Murakami A, Akamine R, Murata K, Nishitani K, Ito H, Watanabe R, Fujii T, Iwasaki T, Masuo Y, Iri O, Nakamura S, Kuriyama S, Morita Y, Murakawa Y, Terao C, Okada Y, Hashimoto M, Matsuda S, Ueno H, Yoshitomi H. Single-cell RNA-seq analysis of synovial CD4+ T cells identifies a novel biomarker and therapeutic target in human rheumatoid arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/single-cell-rna-seq-analysis-of-synovial-cd4-t-cells-identifies-a-novel-biomarker-and-therapeutic-target-in-human-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/single-cell-rna-seq-analysis-of-synovial-cd4-t-cells-identifies-a-novel-biomarker-and-therapeutic-target-in-human-rheumatoid-arthritis/
