Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Cutaneous lupus erythematosus (CLE) is an incompletely understood autoimmune disease that can occur in isolation or in the context of SLE. CLE is often disfiguring, and no FDA-approved therapies exist. Further, evidence suggests skin inflammation in CLE can provoke systemic autoimmune disease, including precipitating nephritis. Thus, understanding CLE pathogenesis has great potential to alleviate lupus morbidity and even mortality.
Methods: We used single-cell RNA sequencing (scRNA-seq) to investigate cellular transcriptomes in lesional skin, sun-protected nonlesional skin, and peripheral blood mononuclear cell (PBMC) samples from 7 CLE patients. 6 of 7 patients met ACR criteria for SLE. Data were analyzed in combination with control skin biopsies taken from 14 healthy patients and PBMCs from 4 healthy donors using Seurat. Keratinocytes (KCs), melanocytes, eccrine gland cells, endothelial cells, fibroblasts (FBs), smooth muscle cells, nerve cells, T cells, myeloid cells, and mast cells were identified. KCs, FBs, T cells, and myeloid cells were sub-clustered and cell subsets annotated. Cytokine module scores were calculated for KCs and FBs using signature genes induced upon treatment of KCs and FBs with panels of cytokines. Cell-cell communication was examined using CellPhoneDB to perform ligand-receptor analysis for select cell subsets. To further investigate cellular interactions, spatial sequencing was performed, and cell signatures derived from scRNA-seq were used to identify the proximity of different cell types in lesional skin from a patient with DLE. Finally, pseudotime analysis was performed on cells bridging the connection between circulating and skin-resident myeloid cells.
Results: Both lesional and nonlesional SLE/CLE KCs showed very strong type I IFN signature scores (Fig. 1). Lesional and nonlesional SLE/CLE skin exhibited dramatic shifts in cell-cell crosstalk (Fig. 2A-C); CD16+ DCs were very enriched in CLE lesions relative to control and were among the most active communications both as expressers of ligands (horizontal) and receptors (vertical) even in nonlesional SLE/CLE skin (Fig. 2D). Spatial transcriptomics demonstrated CD16+ DCs localizing most prominently in the superficial dermis, enabling interaction with KCs. Sub-clustering of paired circulating and skin myeloid cells revealed CD16+ DCs may arise from non-classical monocytes, with discrete shifts in myeloid cell transcriptional states, including a robust IFN education in the skin, detectable across this transition (Fig. 3).
Conclusion: Nonlesional skin of patients with SLE and CLE exists in a type I IFN-rich, “prelesional” state. This affects gene transcription in all major cell types present in skin and dramatically alters cell-cell communication. Non-classical monocytes may infiltrate this environment to become CD16+ DCs that engage in crosstalk with diverse cell types as one of the earliest steps in the evolution of CLE lesions.
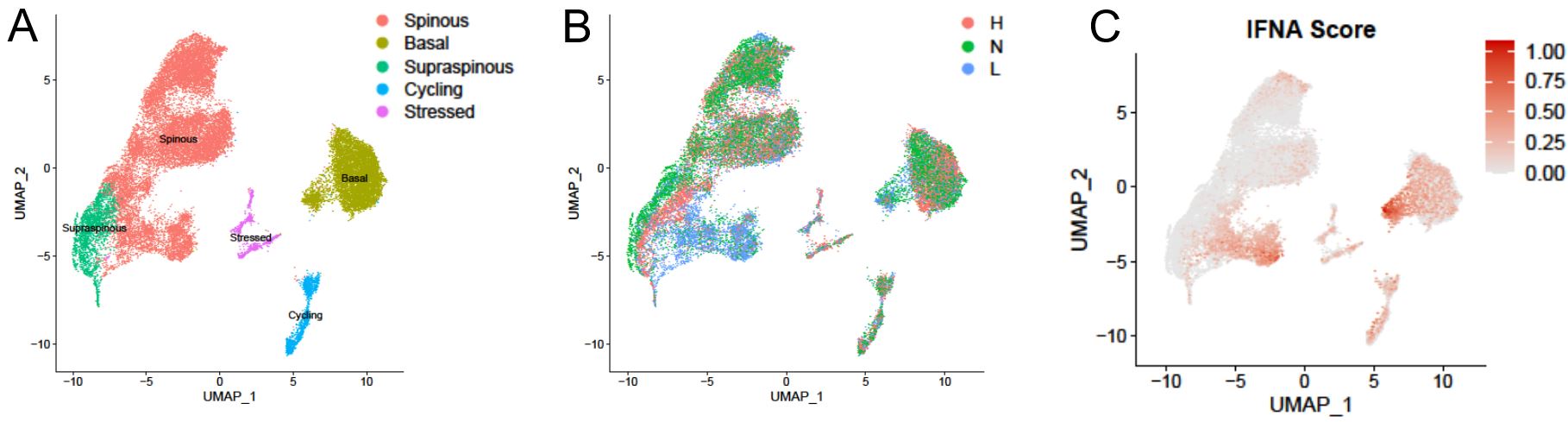 Fig. 1. KC sub-clustering. A. KC subsets. B. KCs colored by origin; healthy (H), nonlesional lupus (N), lesional lupus (L) skin. C. KCs colored by IFNα cytokine module scores.
Fig. 1. KC sub-clustering. A. KC subsets. B. KCs colored by origin; healthy (H), nonlesional lupus (N), lesional lupus (L) skin. C. KCs colored by IFNα cytokine module scores.
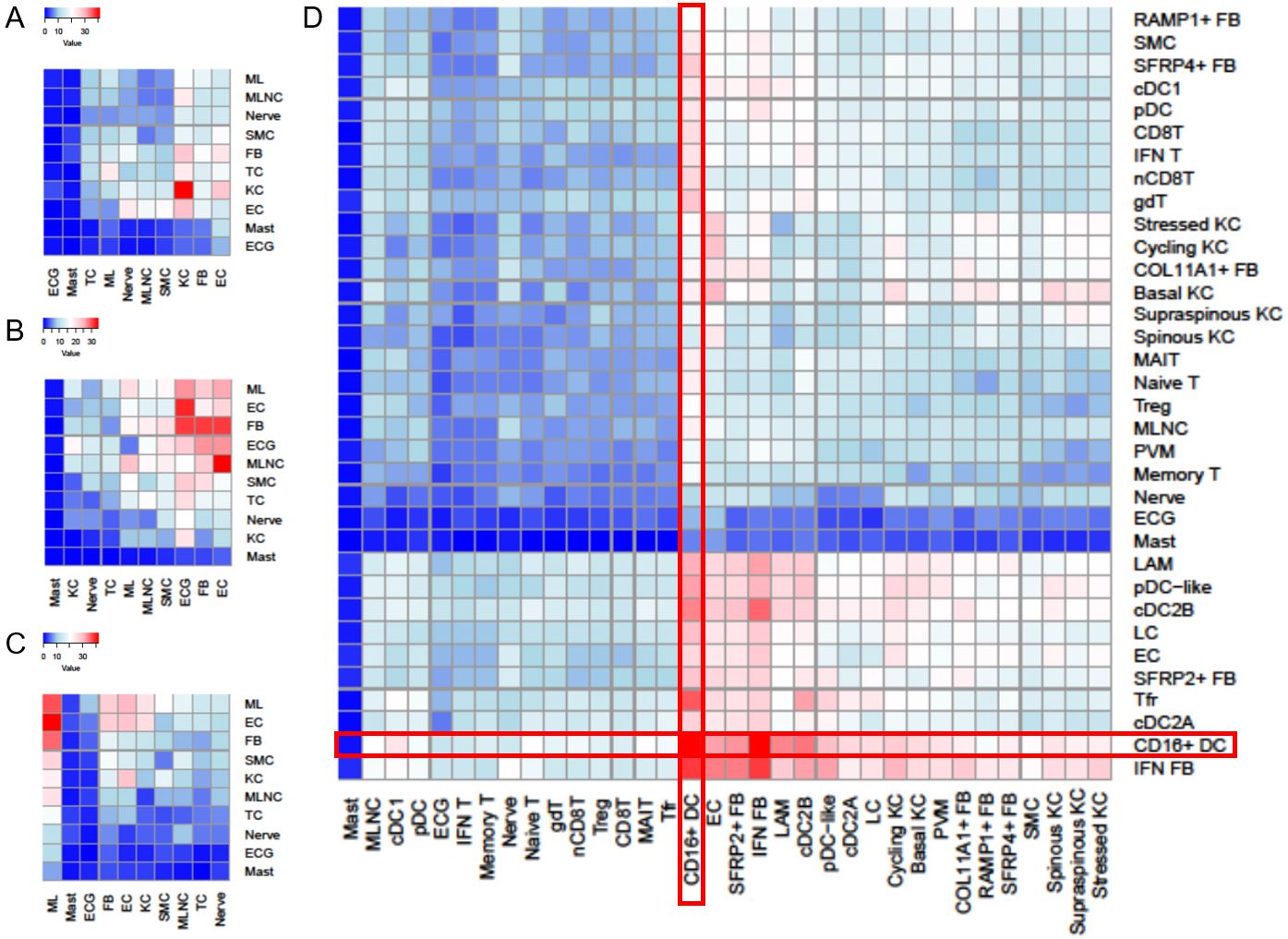 Fig. 2. Number of predicted ligand-receptor interactions in skin. Red, more interactions. ML, myeloid cell. MLNC, melanocyte. SMC, smooth muscle cell. TC, T cell. EC, endothelial cell. ECG, eccrine gland. A. Control skin. B. Nonlesional lupus skin. C. Lesional lupus skin. D. Receptor-ligand interactions of detailed cell subsets in the skin.
Fig. 2. Number of predicted ligand-receptor interactions in skin. Red, more interactions. ML, myeloid cell. MLNC, melanocyte. SMC, smooth muscle cell. TC, T cell. EC, endothelial cell. ECG, eccrine gland. A. Control skin. B. Nonlesional lupus skin. C. Lesional lupus skin. D. Receptor-ligand interactions of detailed cell subsets in the skin.
 Fig. 3. CD16+ DCs may arise from circulating non-classical monocytes (ncMo). A. Myeloid cells from paired skin and PBMC samples colored by subset. Mono, classical monocyte. B. Colored by origin. HP, healthy PBMC. LP, lupus PBMC. C. Pseudotime projection of the transition of ncMo to CD16+ DC. D. Gene markers cluster into five expression patterns across pseudotime.
Fig. 3. CD16+ DCs may arise from circulating non-classical monocytes (ncMo). A. Myeloid cells from paired skin and PBMC samples colored by subset. Mono, classical monocyte. B. Colored by origin. HP, healthy PBMC. LP, lupus PBMC. C. Pseudotime projection of the transition of ncMo to CD16+ DC. D. Gene markers cluster into five expression patterns across pseudotime.
To cite this abstract in AMA style:
Billi A, Ma F, Plazyo O, Hile G, Xing X, Gharaee-Kermani M, Wasikowski R, Tsoi L, Pellegrini M, Modlin R, Gudjonsson J, Kahlenberg J. Single-cell Analysis of Paired Skin and Blood Samples from Patients with SLE and Cutaneous Lupus Suggests CD16+ DCs Arise from Non-classical Monocytes That Enter Nonlesional Skin, Undergo Type I IFN Education, and Engage in Extensive Crosstalk with Diverse Immune and Stromal Cell Types [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/single-cell-analysis-of-paired-skin-and-blood-samples-from-patients-with-sle-and-cutaneous-lupus-suggests-cd16-dcs-arise-from-non-classical-monocytes-that-enter-nonlesional-skin-undergo-type-i-ifn-e/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/single-cell-analysis-of-paired-skin-and-blood-samples-from-patients-with-sle-and-cutaneous-lupus-suggests-cd16-dcs-arise-from-non-classical-monocytes-that-enter-nonlesional-skin-undergo-type-i-ifn-e/
