Session Information
Date: Sunday, November 17, 2024
Title: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) is a clinician-scored instrument that measures activity and damage in the skin of Dermatomyositis (DM) patients. Despite favorable validity, reliability, and excellent responsiveness, the CDASI requires significant expertise and training. Additionally, there may be substantial inter-rater variability in the evaluation of different shades of erythema, especially in darker skin tones, as well as in the evaluation of scales, and ulcerations. Our study aims to validate simplified versions of the CDASI activity score.
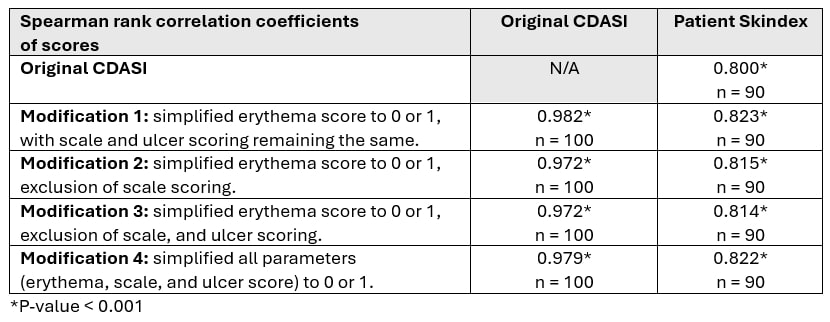
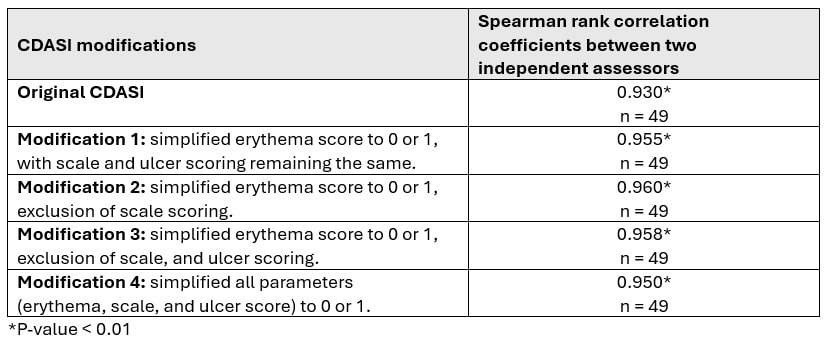
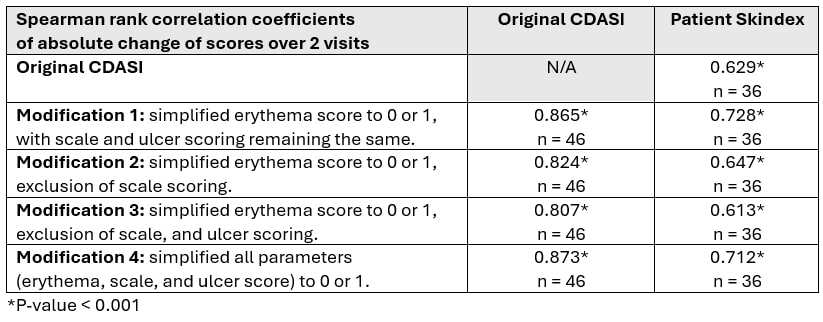
Methods: DM patients satisfying the ACR/EULAR 2017 classification criteria, were prospectively enrolled between August 2022 and April 2024 at the University of Pittsburgh Myositis Center. Patients were assessed by two independent rheumatologists using the CDASI (score 0 – 100). Additionally, patients completed the Skindex questionnaire to assess skin-related quality of life. Four modifications were made to the original CDASI activity score: (1) modification 1: previous erythema score noting absent, pink, red, dark red (0 – 3) to erythema absent/present (0 – 1) with no change in scale and ulcer scoring; (2) modification 2, simplified erythema score and exclusion of scale scoring; (3) modification 3, simplified erythema score, exclusion of scale and ulcer scoring; (4) modification 4, simplification of all parameters (erythema, scales and ulcer score) to 0 – 1 (absent, present).
Results: 27 DM patients [81.5% female, 96.3% Caucasian; median (IQR) age 50.0 (38.0 – 61.0) years and a median (IQR) disease duration 38.0 (9.5 – 88.0) months] with 1-2 clinic visits spaced at least 3 months apart were enrolled. The median (IQR) CDASI activity was 4.5 (1.0 – 12.0) and the score ranges for the aforementioned 4 CDASI modifications were 0 – 66 (modification 1), 0 – 36 (modification 2), 0 – 20 (modification 3), and 0 – 50 (modification 4). All CDASI scoring modifications correlated strongly with the original CDASI and Skindex, indicating good convergent validity. Inter-rater reliability for all modifications was high, as indicated by strong correlations between assessors. The absolute changes from baseline in CDASI score modifications correlated strongly with absolute changes from baseline in original CDASI scores and Skindex, demonstrating good responsiveness.
Conclusion: A simplified CDASI scoring system demonstrated favorable validity, reliability, and responsiveness in the longitudinal evaluation of rash in DM patients. These simple modifications could enable the use of this valuable instrument among more physician subspecialists caring for and monitoring skin disease activity in DM.
To cite this abstract in AMA style:
Pongtarakulpanit N, Chandra T, keret s, Gkiaouraki E, Liarski V, Ascherman D, Mogahadam S, Oddis C, Aggarwal R. Simplified Cutaneous Dermatomyositis Disease Area and Severity Index Activity Scores for Cutaneous Dermatomyositis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/simplified-cutaneous-dermatomyositis-disease-area-and-severity-index-activity-scores-for-cutaneous-dermatomyositis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/simplified-cutaneous-dermatomyositis-disease-area-and-severity-index-activity-scores-for-cutaneous-dermatomyositis/