Session Information
Date: Tuesday, November 12, 2019
Title: 5T087: Edmond L. Dubois, MD Memorial Lecture: SLE – Basic Science (2733–2737)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Systemic lupus erythematosus (SLE) is a multisystemic autoimmune disease characterized by quantitative and qualitative deficiencies of immune cells. Natural killer (NK) cells are innate lymphoid cells that play an important role in the interaction between innate and adaptive immune systems and contribute to the elimination of infected, damaged and tumor cells. Altered NK function is one of the features that characterizes human SLE and lupus prone mice. Despite recent progress into the understanding of NK cells in SLE, the cellular and molecular mechanisms involved in their dysfunction remain elusive. In this context, we carried out an exhaustive analysis of SLE NK cells.
Methods: Cryopreserved peripheral blood mononuclear cells (PBMC) from 24 SLE patients and gender-, age- and ethnicity matched controls were examined by mass cytometry (CyTOF). A panel of 40 markers comprising the conventional inhibitory and activator molecules was designed to characterize NK cells. Cytolytic enzyme expression, cytokine production and degranulation were examined by flow cytometry. Different type of stimulations were used in the above experiments to activate NK cells: cytokines mix, monoclonal antibodies, phorbol 12-myristate-13-acetate/ionomycin and/or K562 and P815 tumor cells.
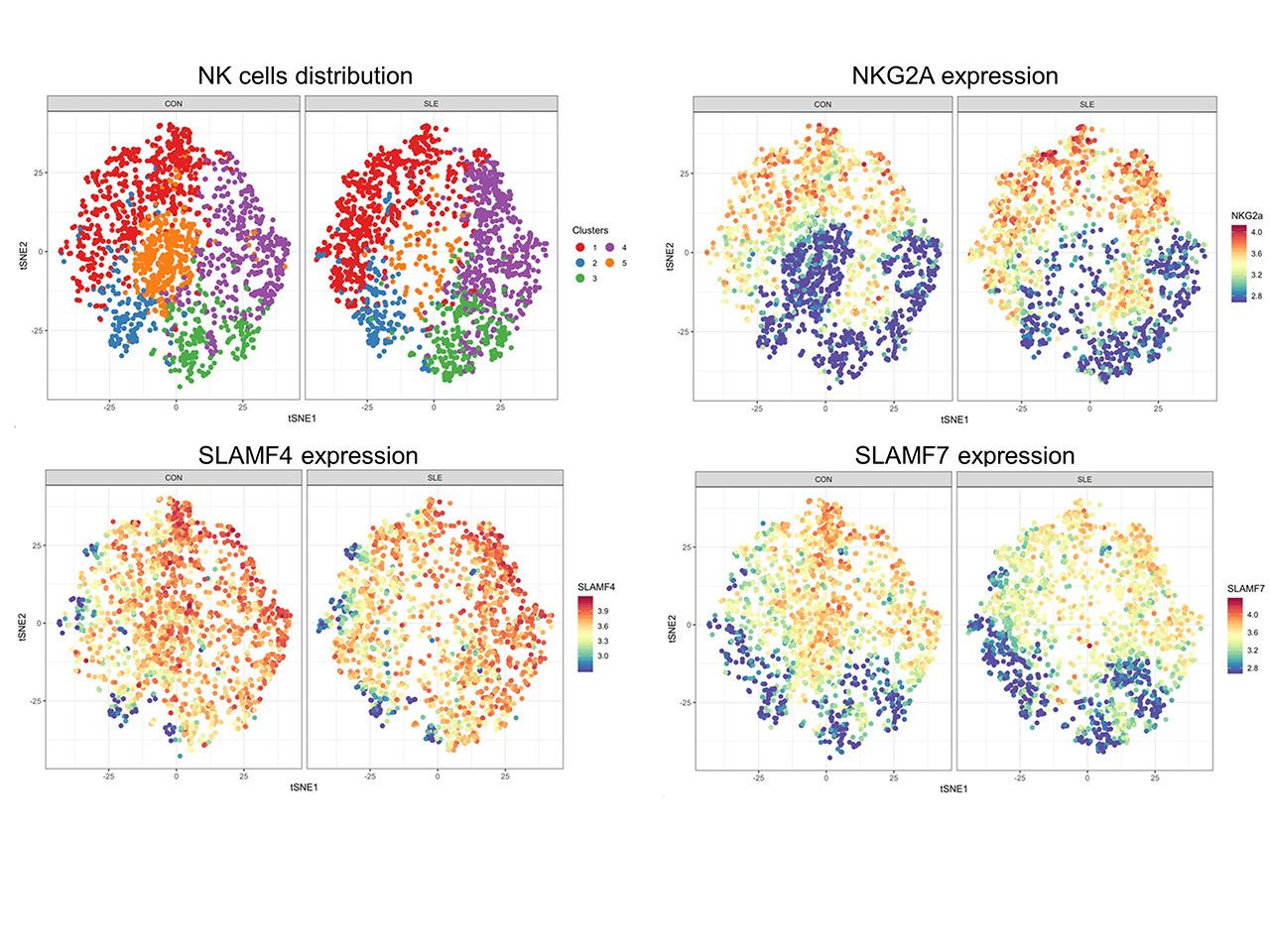
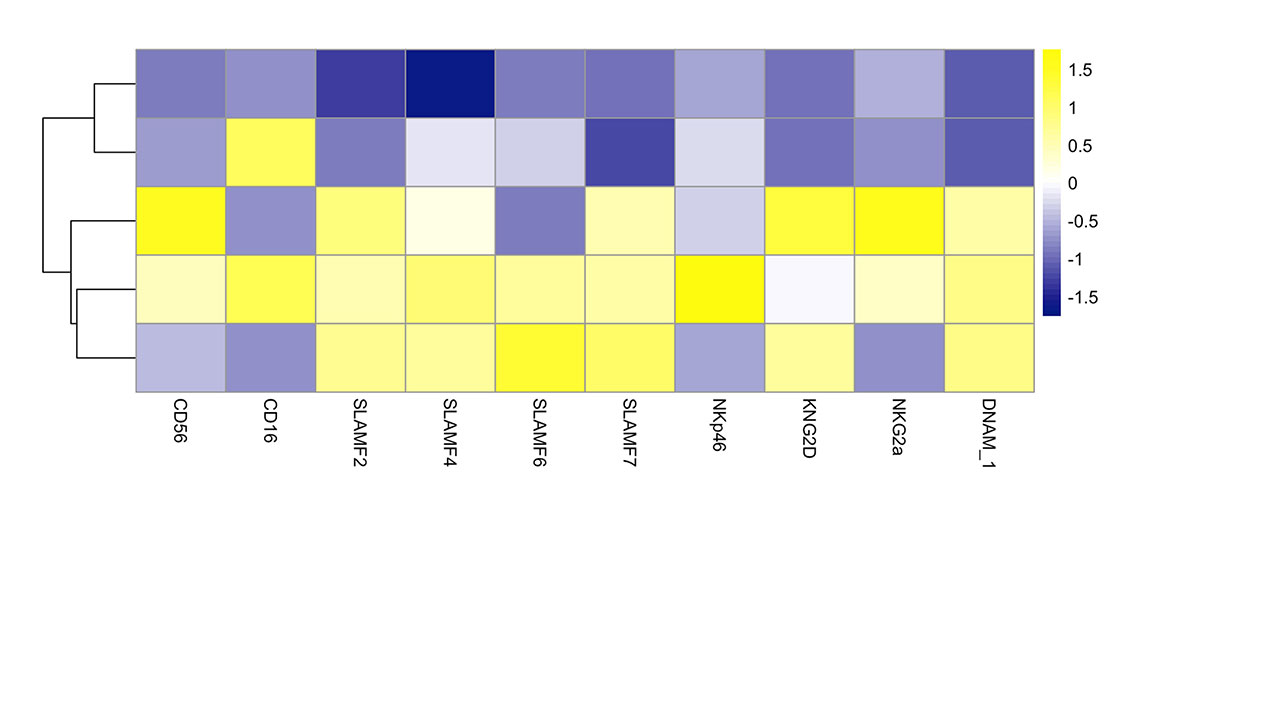
Results: As previously described, our results confirmed a significant decrease in the number and frequency of NK cells in the peripheral blood of SLE patients compared to controls. SLE NK cells exhibited altered functional properties. We observed that cytokine production, cell degranulation and cytotoxic lysis of target tumor cells were reduced. Bioinformatic analysis of mass cytometry data permitted to detect subtle alterations in the expression of phenotypic markers in SLE NK cells discerning them from control NK cells.
This comprehensive analysis emphasized that SLE NK cells display an aberrant expression of receptors belonging to the Signaling Lymphocytic Activation Molecules Family (SLAMF), a group of 8 cell surface receptors, involved in the regulation of immune cells activation. This dysregulation concerns mainly SLAMF1 and SLAMF7, which are respectively positively and negatively regulated on the surface of activated SLE NK cells compared to healthy controls. Further examination of SLAMF1 and SLAMF7 with confocal microscopy show that their distribution is altered on SLE NK cell surface.
Conclusion: Our data confirmed that NK cells isolated from the peripheral blood of SLE patients are dysfunctional, as cytokine production, degranulation and cytotoxic response are compromised. This comprehensive analysis of NK cell surface markers was designed to identify an immune signature characterizing SLE NK cells and permit to demonstrate that SLAMF receptors dysregulation plays a major role in the impairment of SLE NK cells.
To cite this abstract in AMA style:
Humbel M, Bellanger F, Fenwick C, Horisberger A, Ribi C, Comte D. Signaling Lymphocytic Activation Molecule Family (SLAMF) Receptors Deregulation Is Implicated in the Altered Function of NK Cells in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/signaling-lymphocytic-activation-molecule-family-slamf-receptors-deregulation-is-implicated-in-the-altered-function-of-nk-cells-in-systemic-lupus-erythematosus/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/signaling-lymphocytic-activation-molecule-family-slamf-receptors-deregulation-is-implicated-in-the-altered-function-of-nk-cells-in-systemic-lupus-erythematosus/