Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Most osteoarthritis (OA) clinical trial protocols require a 0-10 pain visual numeric scale (VNS) or other quantitative pain score of 4/10 or more for inclusion. Higher pain scores provide a statistically greater possibility for improvement, but also are seen in patients with a higher likelihood of fibromyalgia (FM) and/or depression, who generally have poorer responses to OA therapies. We analyzed OA patients seen in routine care with different levels of pain VNS scores for the proportion who screened positive for FM and/or depression according to FAST3F (fibromyalgia assessment screening tool-fatigue), which agrees ~90% with revised 2011 FM criteria and does not include a pain VNS, and/or MDS2 (MDHAQ depression screen), which agrees ~80% with reference PHQ-9 and HADS-D depression scales, within a single 2-page MDHAQ (multidimensional health assessment questionnaire).
Methods: The MDHAQ is a 2-page patient self-report questionnaire designed for routine care, given to all patients at all visits at the 2 rheumatology settings studied, completed by most patients in fewer than 10 minutes. The MDHAQ includes a 0-10 pain VNS, 0-10 fatigue VNS, 0-3.3 depression query, self-report 0-54 patient painful joint count, 60-symptom checklist including depression, medical history queries, and FAST3F and MDS2 indices. FAST3F is positive (POS) for FM if 2/3 of: fatigue VNS ≥6/10, self-report painful joint count ≥16/54, and/or symptom checklist ≥16/60. MDS2 is POS for depression if 0-3.3 depression query is ≥2.2 OR depression is checked on the symptom checklist. Cross-tabulations of pain VNS vs the proportion of patients who screened POS or negative (NEG) for FAST3F and/or MDS2 were analyzed by chi square tests.
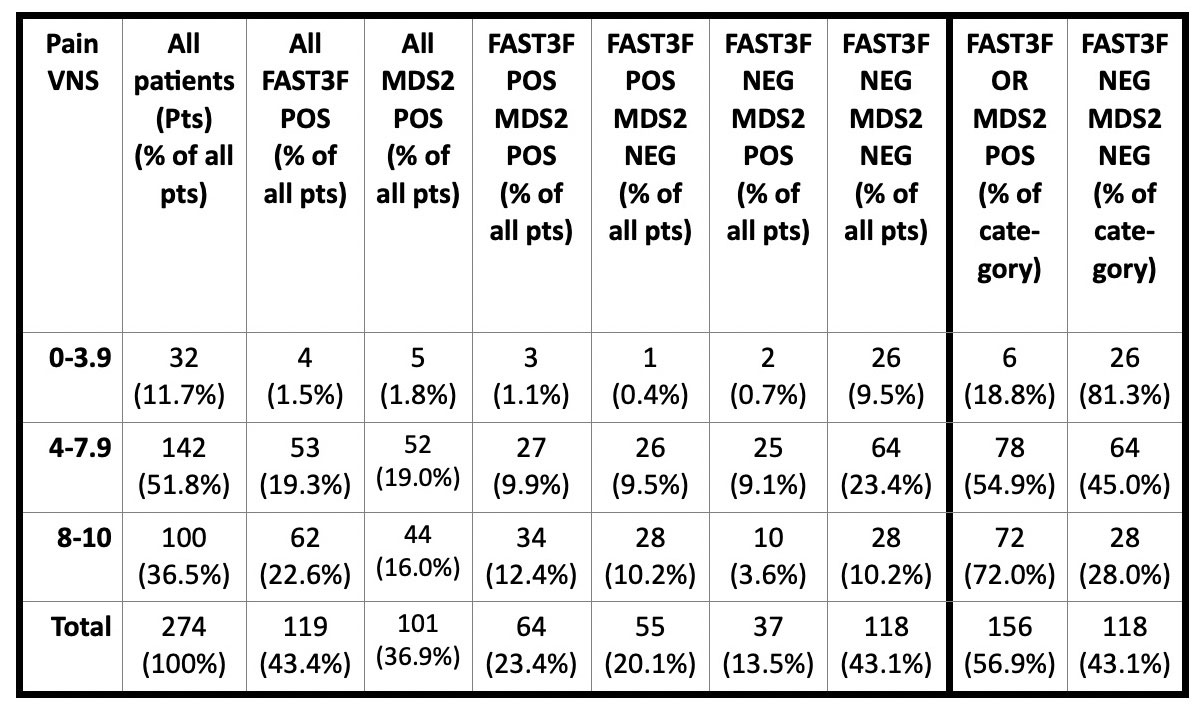
Results: 274 patients with a primary diagnosis of OA were studied at 2 settings; results from the 2 settings were similar and pooled. Of the 274 patients, 156 (56.9%) were POS for either FAST3F or MDS2, including 64 (23.4%) for both FAST3F and MDS2, 55 (20.1%) for only FAST3F, 37 (13.5%) for only MDS2, and 118 (43.1%) for neither FAST3F nor MDS2 (Table). Overall, 32 patients (11.7%) reported pain VNS 0–3.9, 142 (51.8%) pain VNS of 4–7.9 and 100 (36.5%) pain VNS of 8–10 (Table). Among 32 patients with pain VNS 0-3.9, 6 (18.8%) were POS for FAST3F and/or MDS2; among 142 with pain VNS 4–7.9, 78 (54.9% were POS for FAST3 F and/or MDS2; among 118 with pain VNS 8–10, 72 (72%) were POS for FAST3F and/or MDS2 Chi-square = 28.5, p< 0.001 (Table).
Conclusion: 72% of patients with pain VNS of 8–10 screen positive for fibromyalgia and/or depression, compared to 54.9% of those with VNS 4–7.9 and 18.8% of those with VNS 0–3.9. Most OA protocols exclude subjects with a diagnosis of FM. We suggest that patients with pain VNS≥ 8, with substantially reduced likelihood of pain improvement, might be analyzed separately or excluded from future protocols to assess a treatment effect of an agent on OA more sensitively. Protocols also might consider patients with pain scores of 2-4/10, who may have a higher likelihood of a response based on the absence of fibromyalgia and/or depression.
To cite this abstract in AMA style:
Schmukler J, Gibson K, Li T, Block J, Pincus T. Should Osteoarthritis Patients with Pain Scores More Than 8/10 Be Analyzed Separately or Excluded from Clinical Trial Protocols, as 72% Screen Positive for Fibromyalgia And/or Depression on a Multidimensional Health Assessment Questionnaire (MDHAQ)? [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/should-osteoarthritis-patients-with-pain-scores-more-than-8-10-be-analyzed-separately-or-excluded-from-clinical-trial-protocols-as-72-screen-positive-for-fibromyalgia-and-or-depression-on-a-multidime/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/should-osteoarthritis-patients-with-pain-scores-more-than-8-10-be-analyzed-separately-or-excluded-from-clinical-trial-protocols-as-72-screen-positive-for-fibromyalgia-and-or-depression-on-a-multidime/