Session Information
Date: Monday, November 13, 2023
Title: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Treatment II: PsA
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Limited information exists on participation and study outcomes by sex in randomized controlled trials (RCTs) among patients with psoriatic arthritis (PsA). Through a systematic literature review and meta-analysis, we aimed to compare patient characteristics and efficacy and safety of advanced therapies between male and female patients with PsA participating in RCT.
Methods: We performed a systematic literature search of Medline, Embase and Central databases, and conference abstract archives from January 1, 2000 to June 30, 2022. RCTs that assessed the efficacy of an advanced therapy (biologic or targeted synthetic) in adult participants with PsA were included. Among studies that reported sex-disaggregated results, we extracted information on participants’ baseline characteristics and proportion of participants achieving minimal disease activity (MDA), or meeting the American College of Rheumatology 20 (ACR20) and ACR50 response criteria at the primary endpoint of the study by sex. Random-effect models were used to calculate pooled effects for response in males vs. females for the different classes of advanced therapies.
Results: A total of 52 trials (21,769 participants) were included. The average percentage of male and female participants enrolled was 50.2% and 49.8%, respectively. Only 9 studies (17.3%) reported sex-disaggregated baseline characteristics, 16 studies (30.7%) reported sex-disaggregated efficacy endpoints and 2 studies (3.8%) reported sex-disaggregated safety endpoints.
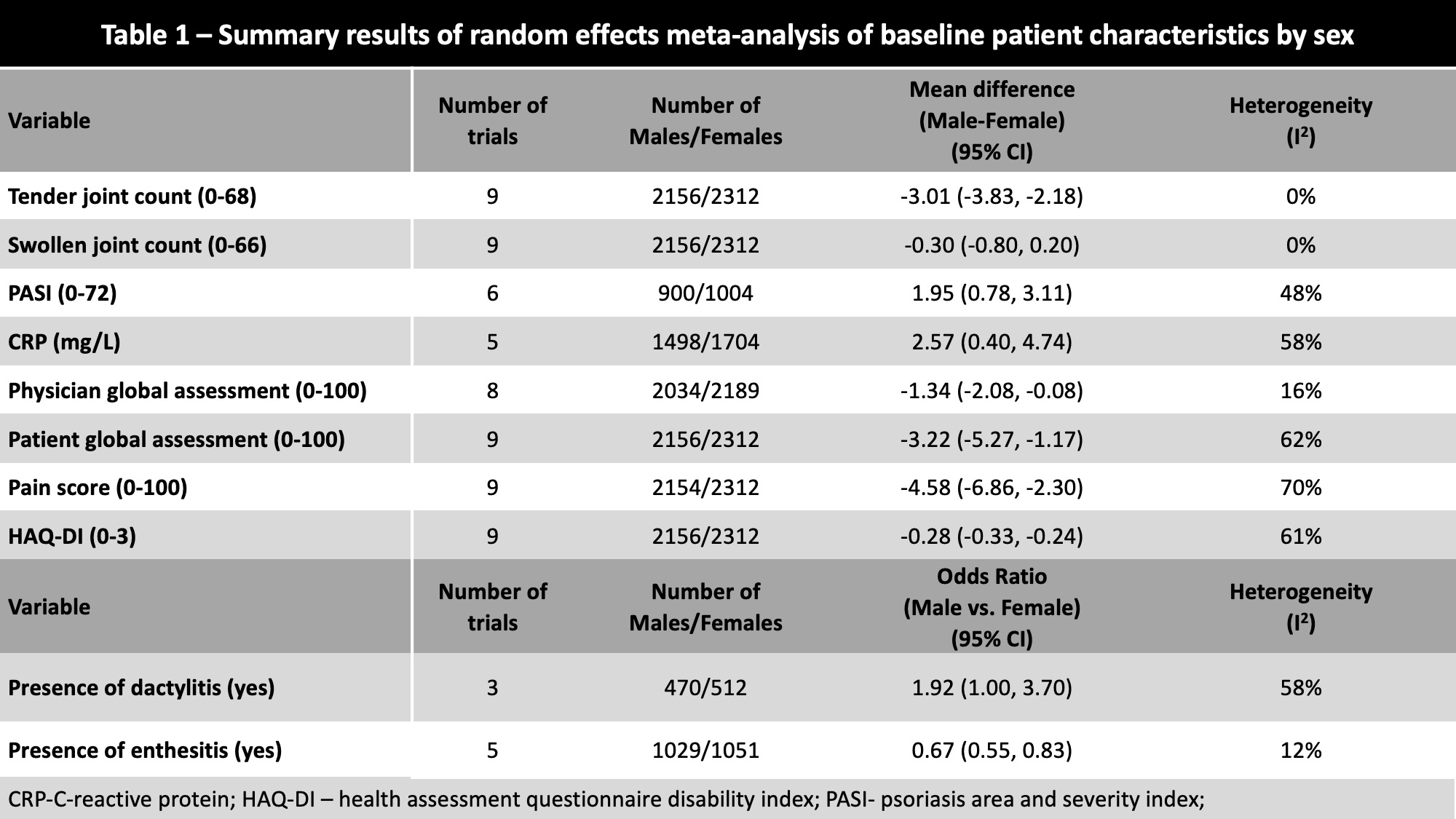
Female patients had significantly higher baseline tender joint count, Health Assessment Questionnaire Disability Index, physician and patient global assessment and pain scores. Male patients had significantly higher baseline psoriasis area and severity index and CRP [Table 1].
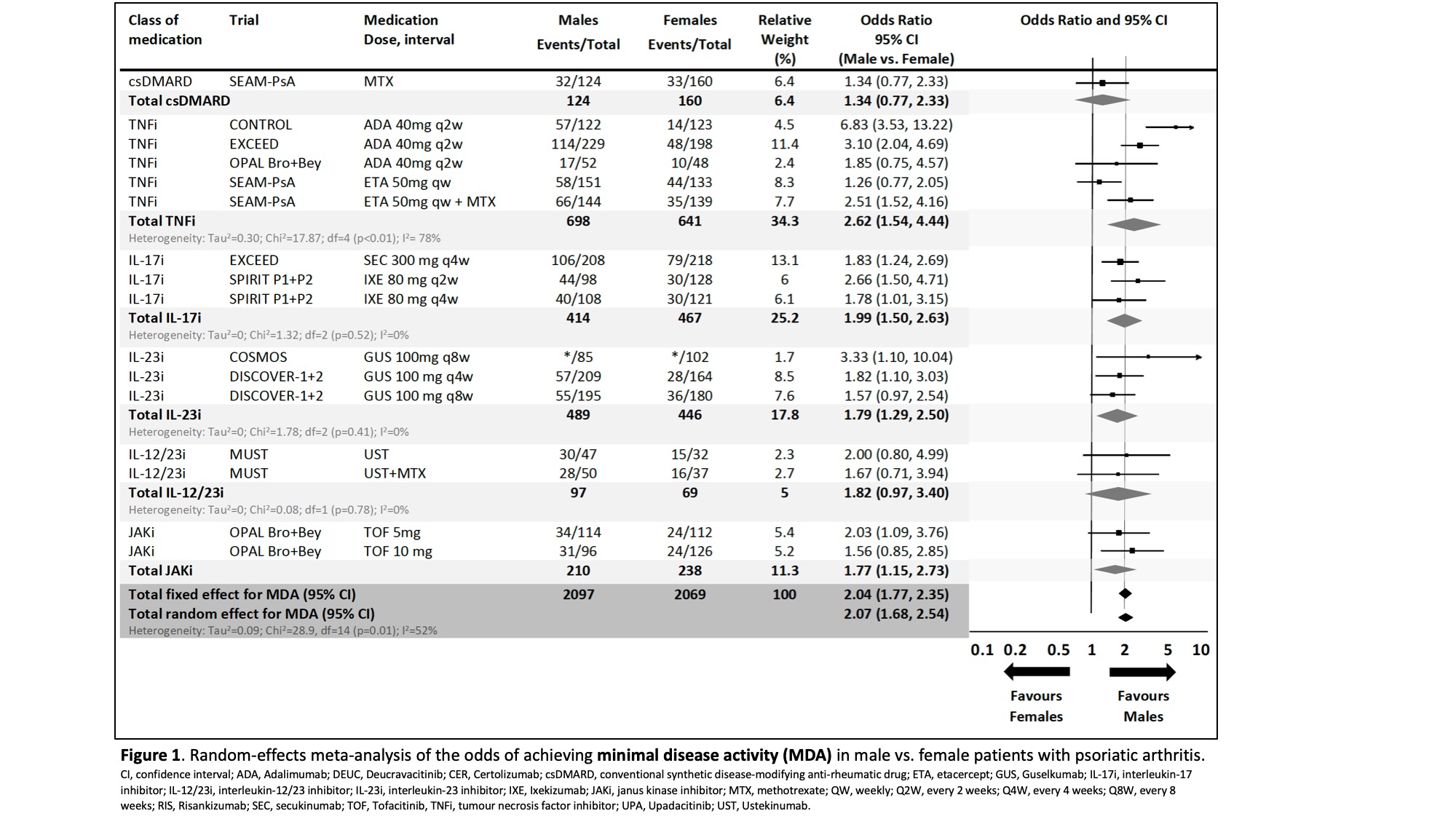
Differences in pooled estimates of efficacy endpoints were seen for male and female patients across the different classes of advanced therapies. The probability of achieving MDA was significantly higher in males in the following classes of advanced therapies [Fig. 1]: IL-17 inhibitors (i) (OR 1.99), IL-23i (OR 1.79), TNFi (OR 2.62) and JAKi (OR 1.77). The probability was also higher in IL-12/23i and methotrexate, but not statistically significant.
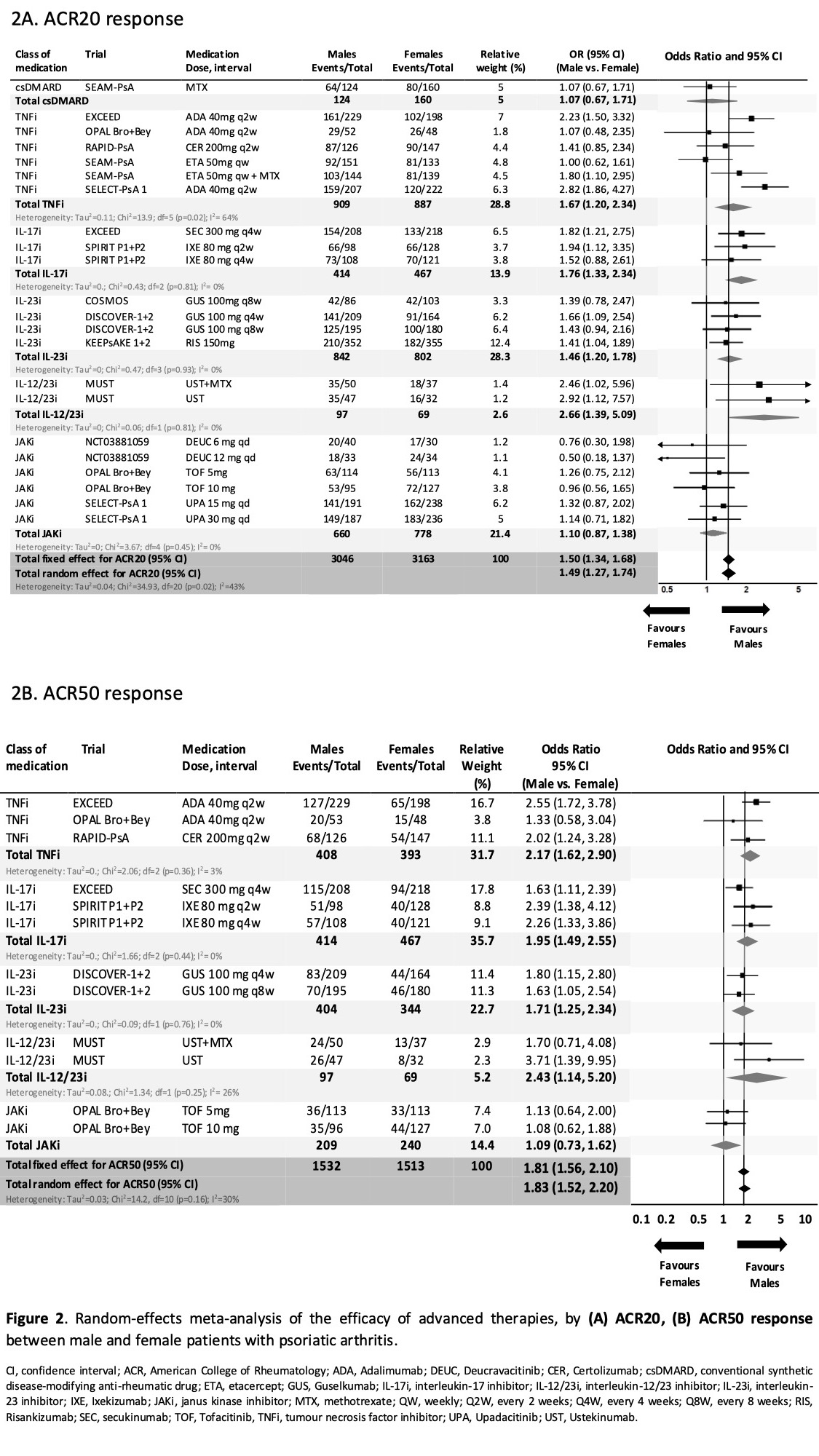
In contrast, variability was seen in the probability of achieving ACR response by sex across classes of advanced therapies. The probability of achieving ACR20 response was significantly higher in male vs. female patients for IL-17i (OR 1.76), IL-23i (OR 1.46), IL-12/23i (OR 2.66) and TNFi (OR 1.67), but significantly different for JAKi (OR 1.10). [Fig. 2A]. Similarly, the probability of achieving ACR50 response was significantly higher in males vs. females in all advanced therapies, and not significantly different for JAKi (OR 1.09) [Fig 2B]. Male and female patients had a similar probability of achieving ACR20 response when using placebo (OR 1.04, 95% CI 0.86, 1.27).
Conclusion: Female patients participating in RCTs are less likely to achieve efficacy end points for most classes of advanced therapies. Some differences in response outcomes were found across classes of advanced therapies. Future studies should report disaggregated sex data for baseline and end-points.
To cite this abstract in AMA style:
Eder L, Mylvaganam S, Pardo Pardo J, Petkovic J, Strand V, Mease P, Colaco K. Sex of the Patient Affects Response to Advanced Therapies in Psoriatic Arthritis: Meta-analysis of Data from Randomized Controlled Trials [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/sex-of-the-patient-affects-response-to-advanced-therapies-in-psoriatic-arthritis-meta-analysis-of-data-from-randomized-controlled-trials/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sex-of-the-patient-affects-response-to-advanced-therapies-in-psoriatic-arthritis-meta-analysis-of-data-from-randomized-controlled-trials/