Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Differences in efficacy outcomes favoring male vs female patients (pts) with RA have been reported with csDMARDs1 and TNF inhibitors;2 results with JAK inhibitors are less clear.3 Here, we assess the impact of sex on efficacy, safety, and persistence in clinical trials of tofacitinib in RA.
Methods: Efficacy and safety were assessed using data from pooled Phase (P)3 RCTs of pts with RA and an inadequate response to MTX (NCT00847613; NCT00853385) or ≥1 DMARD (NCT00856544) who received tofacitinib 5 or 10 mg BID, adalimumab (ADA) 40 mg Q2W, or placebo (PBO; advancing to tofacitinib at Month [M]3/6) + csDMARDs. Persistence was assessed in pts receiving tofacitinib 5 or 10 mg BID ± csDMARDs using data pooled from two LTE trials (NCT00661661; NCT00413699). Efficacy outcomes (to M12): ACR20/50/70 responses, changes (Δ) from baseline (BL) in DAS28-4(ESR), CDAI, CRP, and HAQ-DI, and DAS28-4(ESR) remission (< 2.6). Safety outcomes (to M24) for tofacitinib and ADA: adverse events (AEs), serious AEs (SAEs), severe AEs, discontinuations due to AEs, and AEs of special interest (AESIs). Kaplan-Meier persistence analysis estimated 2- and 5‑year drug survival rates with tofacitinib.
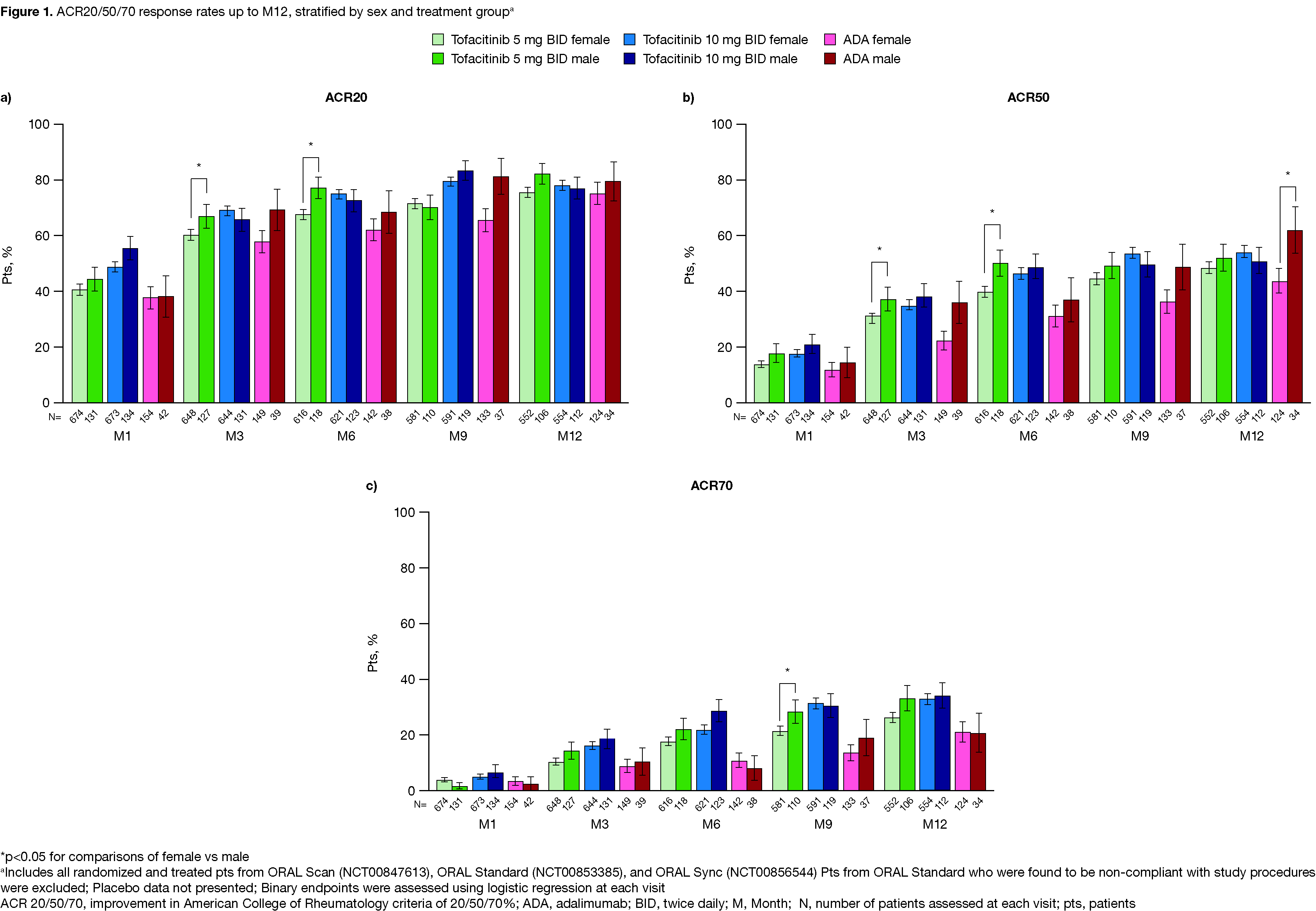
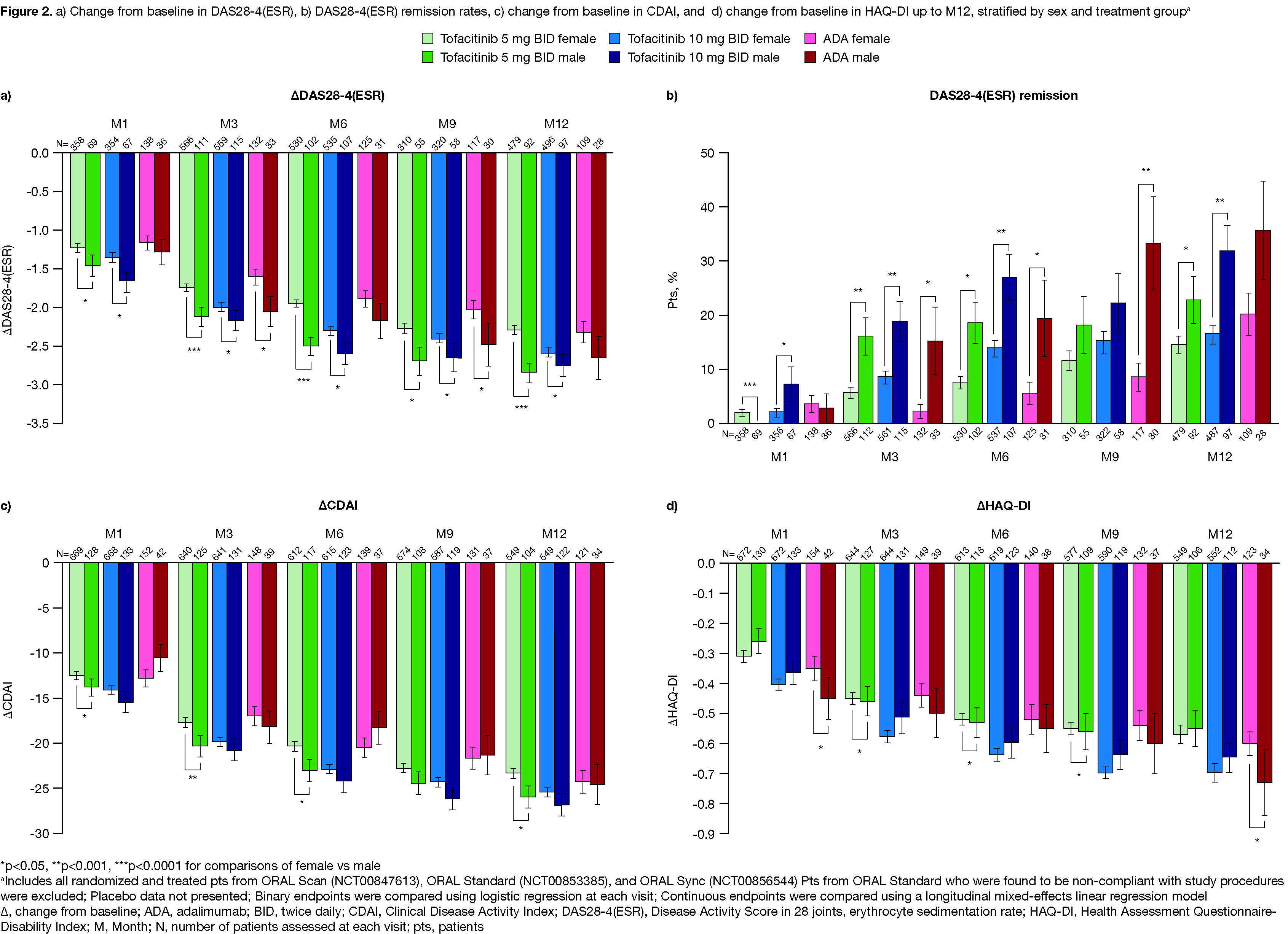
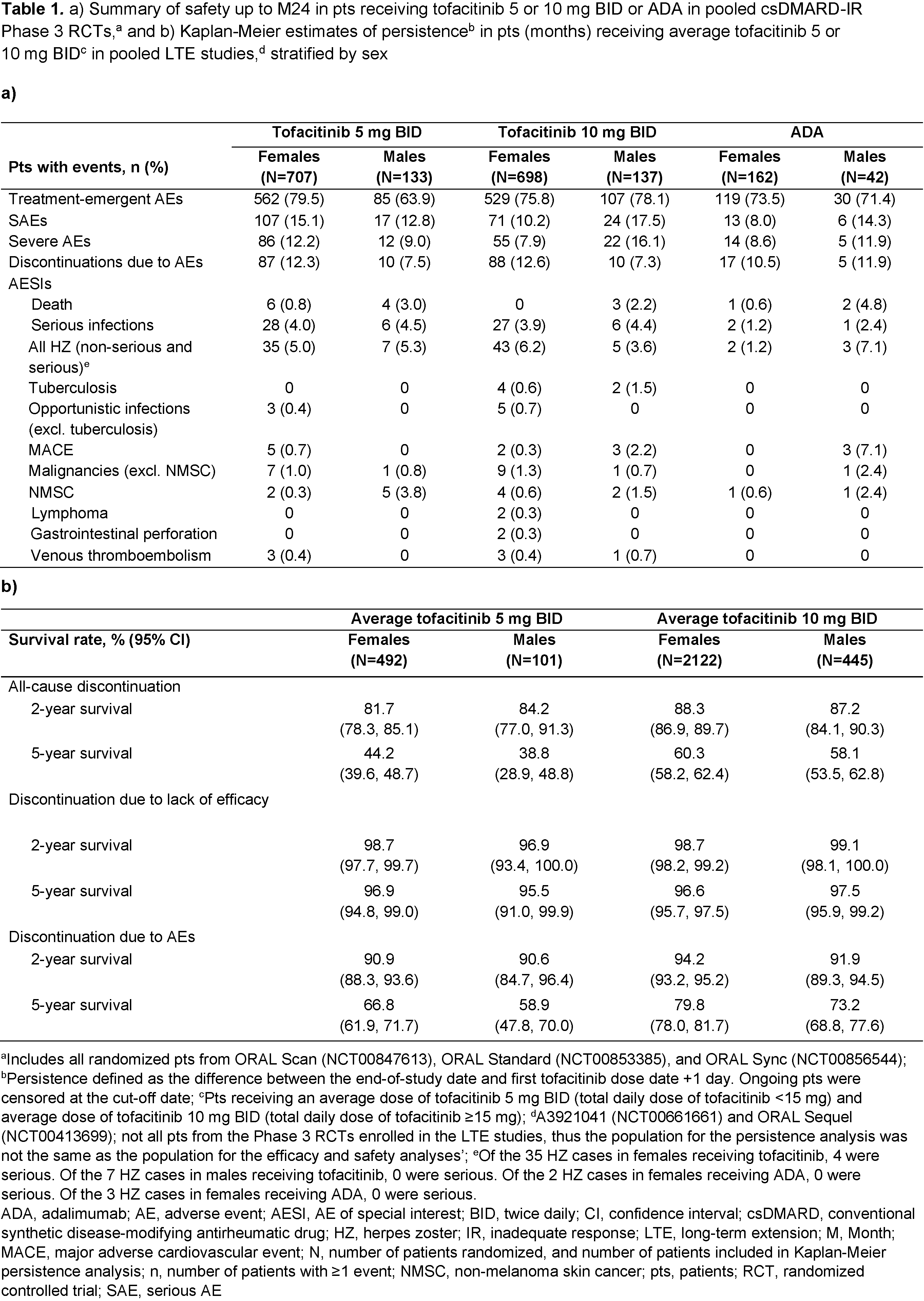
Results: 2,265 pts were included from the P3 RCTs. Demographics and BL characteristics were comparable across sexes and treatments. Tofacitinib or ADA vs PBO generally led to significantly higher ACR20/50/70 responses in both sexes through M6 (data not shown). Up to M12, ACR20/50/70 responses were broadly comparable across active treatments and between sexes, with significant differences favoring males observed at some timepoints (Figure 1). Statistically significant differences favoring males vs females were observed in ΔDAS28-4(ESR) and DAS28-4(ESR) remission rates at most timepoints across treatment groups (Figure 2a,b). Differences in ΔCDAI, ΔCRP (data not shown), and ΔHAQ-DI tended to favor males (except ΔHAQ‑DI with tofacitinib 10 mg BID, which was numerically greater in females) (Figure 2c,d). Rates of AEs, SAEs, severe AEs, and discontinuations due to AEs were slightly higher in females vs males with tofacitinib 5 mg BID (Table 1a); with tofacitinib 10 mg BID and ADA, this trend was generally reversed. AESIs were comparable between sexes with tofacitinib and ADA, although event numbers were low and results should be interpreted with caution. 2‑ and 5-year tofacitinib survival rates were mostly similar between sexes, with some numerical, non-significant differences favoring females (Table 1b).
Conclusion: In this post hoc analysis, efficacy outcomes with tofacitinib and ADA were generally higher in males and comparable in females vs previously reported mixed population response rates for advanced therapies.4,5 Safety findings did not reveal a consistent pattern between sexes. Tofacitinib persistence was similar between sexes.
- Bergstra SA et al. J Rheumatol 2018; 45: 1361-1366.
- Jawaheer D et al. J Rheumatol 2012; 39: 46-53.
- Spinelli FR et al. Ann Rheum Dis 2020; 79: 1016-1017.
- Bird P et al. J Clin Rheumatol 2019; 25: 115-126.
- Rein P, Muller RB. Rheumatol Ther 2017; 4: 247-261.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by Christina Viegelmann, CMC Connect, and funded by Pfizer Inc.
To cite this abstract in AMA style:
Jones N, Strand V, Schulze-Koops H, Mysler E, Kinch C, Gruben D, Germino R, Connell C, Eder L. Sex Differences in the Efficacy and Safety of Tofacitinib in Rheumatoid Arthritis Patients: A Post Hoc Analysis of Phase 3 and Long-Term Extension Trials [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/sex-differences-in-the-efficacy-and-safety-of-tofacitinib-in-rheumatoid-arthritis-patients-a-post-hoc-analysis-of-phase-3-and-long-term-extension-trials/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sex-differences-in-the-efficacy-and-safety-of-tofacitinib-in-rheumatoid-arthritis-patients-a-post-hoc-analysis-of-phase-3-and-long-term-extension-trials/