Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Gastrointestinal (GI) involvement is the most frequent internal complication of systemic sclerosis (SSc) and, when severe, it has a major influence on patient function. Despite SSc being an autoimmune disease and the availability of several immune targeted interventions for Inflammatory bowel diseases, GI involvement has never been directly targeted in clinical intervention trials in SSc. The impact of GI involvement on patient function is properly captured by the GI domains for the SCLERO ID which has shown good correlation with other validated GI PRO such as UCLA GIT 2.0 (Nagy G et al, Arthritis Res Ther, 2023). Here we aimed to identify the distribution of severity of GI involvement in a single centre observational study, to identify the clinical and demographic features associated with severe involvement and to determine the natural history of GI impact on patient function in a longitudinal setting.
Methods: Patients fulfilling ScleroID PROs from the STRIKE cohort were enrolled in this analysis. Patients in the upper quartile distribution for both Upper GI and Lower GI ScleroID were labelled as having high impact from GI involvement. Student’s T and Wilcoxon’s tests were employed for group comparisons as appropriate. Longitudinal changes in the severity scores were visualised through alluvial plots. Statistical analysis was performed using R v. 4.4.0.
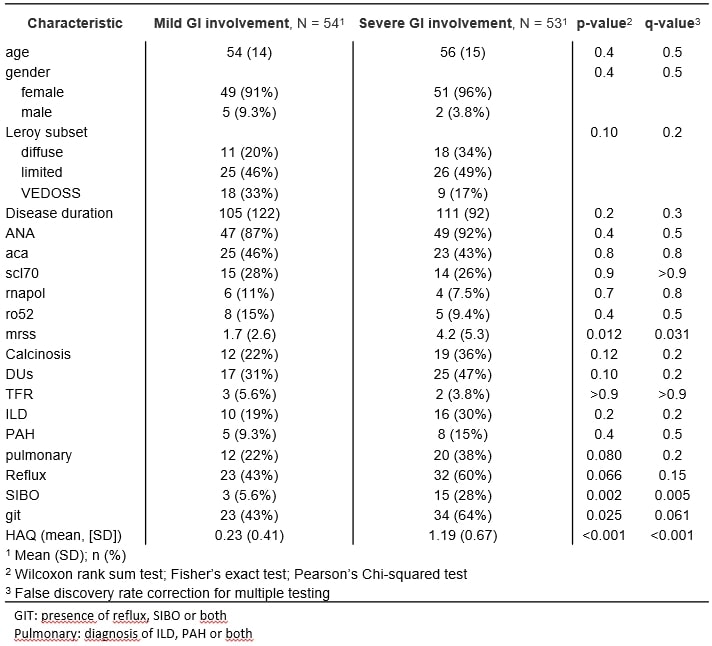
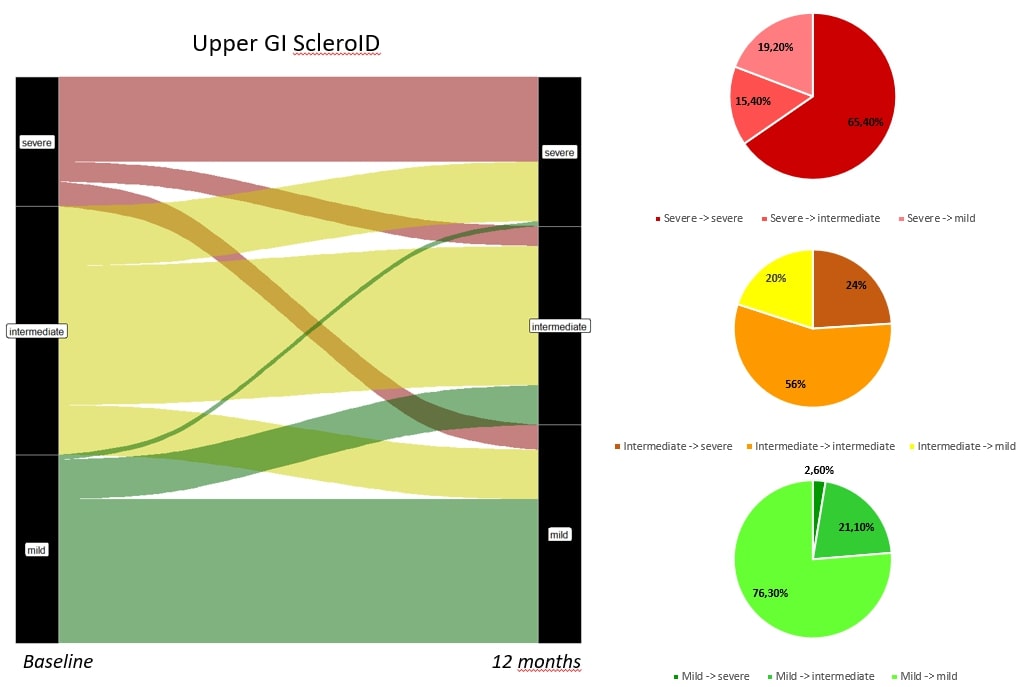
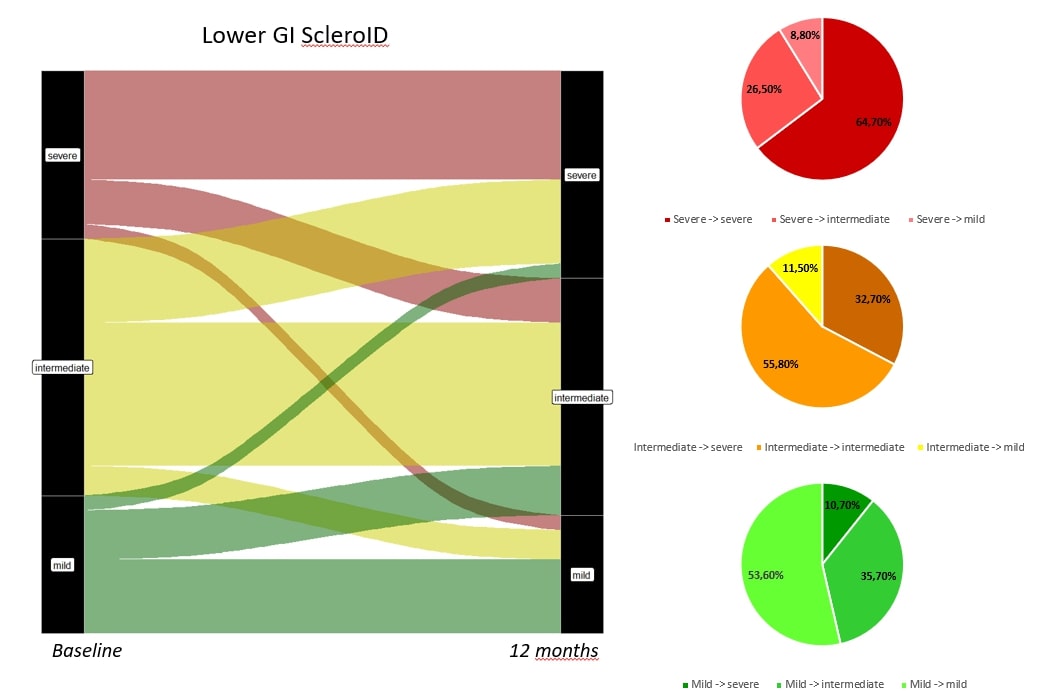
Results: 276 patients were enrolled, 203 fulfilling ACR criteria and 73 with Very early SSc. GI domains of Sclero ID had a not normal distribution with Median (IQR) scores of 3 (1,7) for upper GI and 4 (0,7) for lower GI. Patients in the first and lower quartile were further analysed as extreme phenotypes. 54 out of 276 partecipants (19.6%) had both mild upper GI and lower GI ScleroID score; 53 patients (19.2%) were in both severe quartiles. Patients with severe GI involvement had higher prevalence of Small Intestinal bacterial overgrowth (SIBO, 28% vs 5.6 %, p= 0.002); higher mean (SD) Modified Rodnan Skin Score (mRSS) [4.2 (5.3) vs 1.7 (2.6), p= 0.012]; as well as higher HAQ-DI scores [1.19 (0.67) vs 0.23 (0.41), p= < 0.001]. Beyond these features, patients with severe GI involvement did not have any significant difference in age, gender, cutaneous subset, disease duration or autoantibody profile compared to the mild phenotype (Figure 1). Follow up PRO was available in 114 patients (figure 2,3). Lower GI scores were more sensitive to change compared to the upper GI ones. Only 12/34 patients with severe lower GI involvement improved at 12 months (35%), whereas 20/80 (20%) joined the severe group from the rest of the population, bringing the total proportion of patients in the severe group from 34/114 (30%) to 42/114 (37%) in the 12 months time point (Figure 3).
Conclusion: Patients with severe GI involvement can be identified as a unique population distributed across very early, limited and diffuse cutaneous subsets in SSc. They show worse skin involvement and overall function, have only a poor response to standard of care treatments and their number increases over time. Identification of biological targets in this novel disease subset is highly warranted to inform much needed intervention trials in this under studied subpopulation in SSc.
To cite this abstract in AMA style:
Minerba M, Bixio R, Colak S, Di Donato S, Bissell L, Del Galdo F. Severe Gastrointestinal Involvement in Systemic Sclerosis: A New “Transversal” Subset of Disease to Be Prioritised for Intervention Trials [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/severe-gastrointestinal-involvement-in-systemic-sclerosis-a-new-transversal-subset-of-disease-to-be-prioritised-for-intervention-trials/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/severe-gastrointestinal-involvement-in-systemic-sclerosis-a-new-transversal-subset-of-disease-to-be-prioritised-for-intervention-trials/