Session Information
Date: Sunday, November 7, 2021
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I (0660–0682)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Use of serum urate (SU) as a treatment target and outcome measure has become controversial in light of the American College of Physician Gout Guidelines which advocated a “treat-to-symptom”, rather than a “treat-to-target SU” approach to management. The choice of SU as a treatment target measure implies that achievement of target SU is causally associated with improvement in patient-centered outcomes such as reduction in the number of gout flares. The aim of this study was to assess the causal relationship between achieving target SU and occurrence of gout flares.
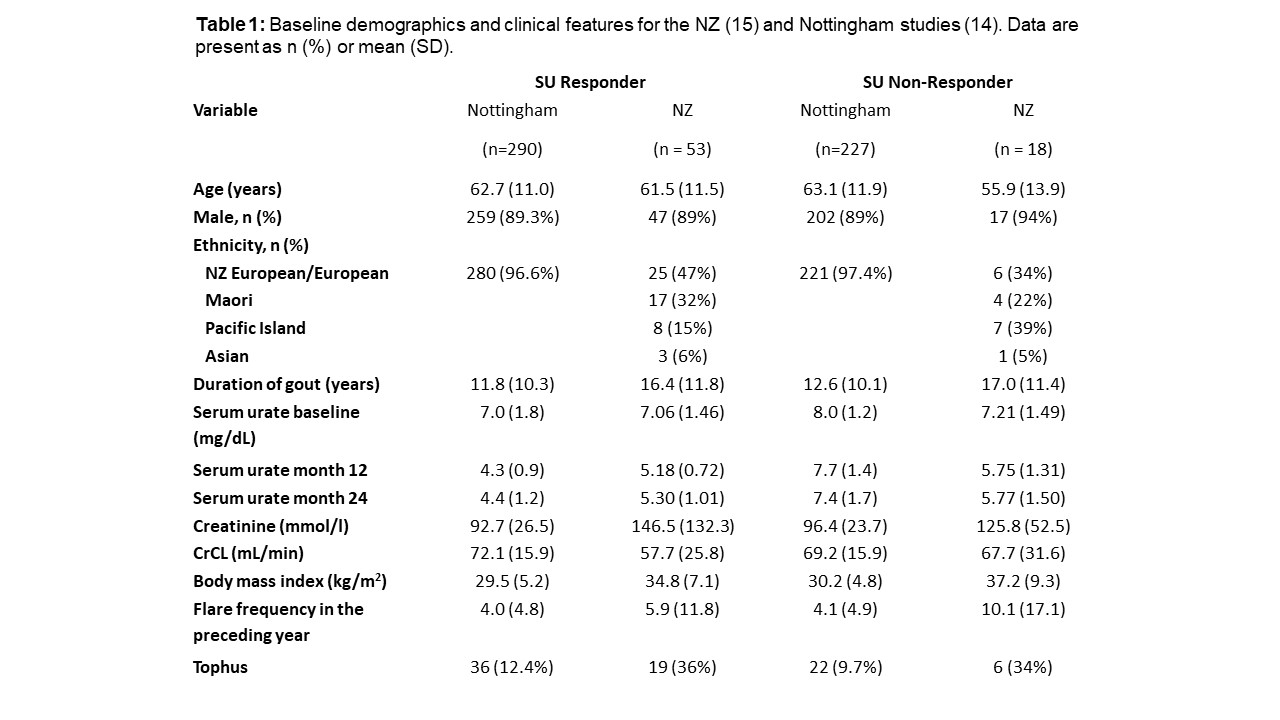
Methods: Individual patient data from two randomized trials were analyzed to determine the relationship between SU and flares. Study one (Nottingham, UK) was a 2-year randomized trial comparing nurse-led and GP-led gout care (n=517) (1). Study two (New Zealand) was a 2-year open label randomized trial assessing the safety and efficacy of allopurinol dose escalation (2, 3). Individuals who on average achieved SU < 6mg/dL at 6, 9 and 12 months post-baseline were defined as “SU responders”. The primary endpoint was the proportion of participants having at least one gout flare from 12 to 24 months. The secondary endpoint was the actual number of flares from 12 to 24 months (collected as a number between 0 and 4 per month) and tophus regression. The individual patient data sets from New Zealand and Nottingham were combined and analyses undertaken for the primary and secondary outcome measures. These analyses were undertaken using the models as above with the addition of a fixed factor to account for New Zealand and randomization within the Nottingham dataset.
Results: Significantly fewer SU responders experienced a gout flare in months 12-24 compared to SU non-responders; unadjusted OR 0.20; 95%CI 0.15 to 0.29. In the Nottingham study, there was no effect of randomization on the relationship between serum urate response group and the presence or absence of gout flares (p=0.13) indicating that the reduction in SU is responsible for the absence of flares. The mean number of flares in months 12-24 was significantly lower in SU responders compared to SU non-responders, unadjusted mean difference -1.64; 95%CI -1.85 to -1.44. This association was also independent of the randomized treatment allocation. Combining the individual data for both NZ and Nottingham revealed those participants with a tophus at baseline, 38/55 (69.1%) of SU responders lost the sentinel tophus compared to 8/22 (36.4%) of the SU non-responders (OR 3.9; 95%CI 1.4 to >11.1).
Conclusion: We have shown using individual patient data from 2 RCTs that achieving an average SU < 6mg/dL over a 6-month period is likely causally associated with the subsequent absence of gout flares, a reduction in the number of gout flares, and resolution of tophi in people with gout during the second year of treatment.
To cite this abstract in AMA style:
Stamp L, Frampton C, Morillon M, Taylor W, Dalbeth N, Singh J, Doherty M, Zhang W, Richardson H, Sarmanova A, Christensen R. Serum Urate Reduction Is Causally Associated with Flare Outcomes in People with Gout: Evidence for Surrogate Status from a Pooled Analysis of 2 Randomized Trials [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/serum-urate-reduction-is-causally-associated-with-flare-outcomes-in-people-with-gout-evidence-for-surrogate-status-from-a-pooled-analysis-of-2-randomized-trials/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-urate-reduction-is-causally-associated-with-flare-outcomes-in-people-with-gout-evidence-for-surrogate-status-from-a-pooled-analysis-of-2-randomized-trials/