Session Information
Date: Monday, November 18, 2024
Title: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Diagnosing hepatocellular injury in idiopathic inflammatory myopathy (IIM) patients with active myositis is challenging due to the lack of liver specificity of the transaminase enzymes. Glutamate dehydrogenase (GLDH) is a liver-specific biomarker capable of detecting hepatocellular injury in patients with muscle injury or degeneration. This study aims to validate serum GLDH levels as a biomarker for liver injury in IIM patients and to help differentiate serum transaminase enzyme elevation due to active myositis.
Methods: We measured serum GLDH levels in stored frozen (-80oC) serum from prospectively enrolled patients in the University of Pittsburgh Medical Center (UPMC) myositis registry and correlated these levels with traditional liver enzyme data collected on the same dates. Hepatocellular liver injury was assessed through clinical judgment based on a review of clinical data, hepatic investigations, and longitudinal changes in liver enzyme patterns over time.
Results: We analyzed 115 serum samples collected at different times from 68 IIM patients (1 – 6 samples per patient). Most of the patients were white (94.8%) and female (62.6%), with a median (IQR) age of 57.0 (45.0 – 65.0) years. Fatty liver was documented in 25.2%. Alcohol use was reported by 42.2% of the patients, and 46.6% had received hepatotoxic drugs. The most common IIM subtype was anti-synthetase syndrome (36.2%), followed by dermatomyositis (20.7%) and immune-mediated necrotizing myopathy (19.0%).
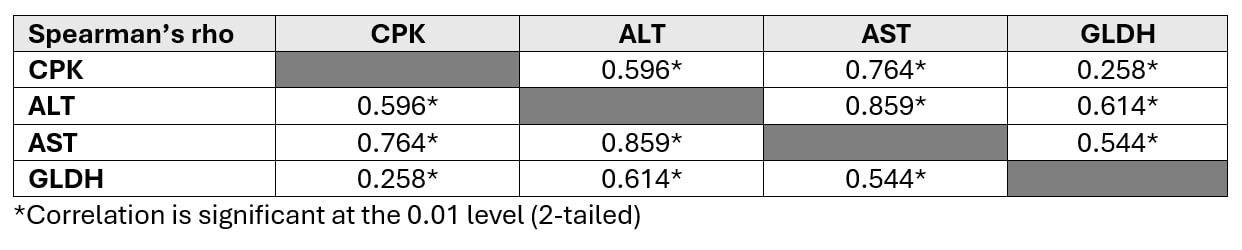
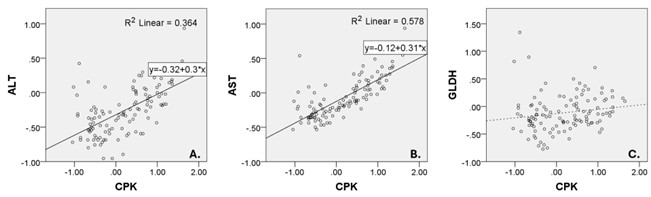
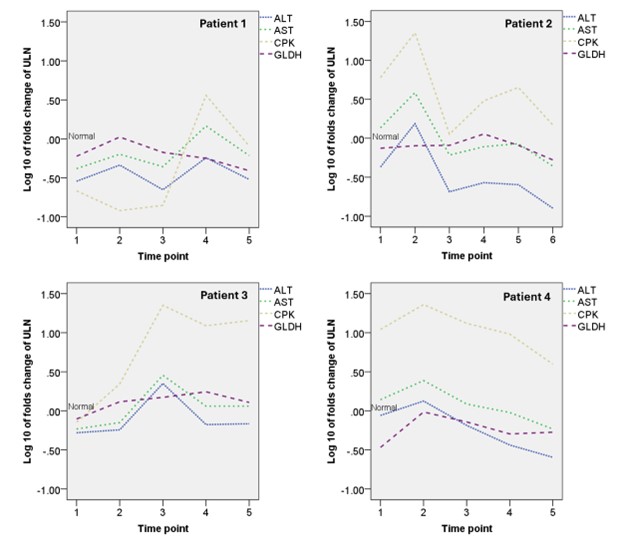
The median (IQR) levels of ALT, AST, and CPK were 32.5 (18.0 – 62.5) IU/L, 31.0 (21.0 – 52.8) IU/L, and 306.0 (66.3 – 1,123.8) IU/L, respectively. The median (IQR) fold increases above the upper limits normal (ULN) for ALT, AST, and CPK were 0.51 (0.29 – 0.97), 0.76 (0.51 – 1.29), and 1.51 (0.33 – 5.71), respectively. Log-transformed fold increases above ULN values of ALT and AST showed a good correlation with CPK (Spearman’s correlation coefficients 0.596 and 0.764, respectively; P < 0.01). In contrast, Log-transformed fold increases above ULN values of GLDH showed a poor correlation with CPK (Spearman’s correlation coefficient 0.258, P < 0.01). There were linear relationships between Log-transformed folds increases above ULN values of CPK and transaminase enzymes but not between the CPK and GLDH. The longitudinal plots demonstrated that GLDH is more specific to liver injury than transaminase enzymes.
Conclusion: In IIM patients, GLDH shows a poor correlation with CPK, confirming its specificity for liver injury independent of muscle inflammation in myositis. GLDH is a specific biomarker for evaluating hepatocellular injury in IIM patients with concurrent myositis.
To cite this abstract in AMA style:
Pongtarakulpanit N, Chandra T, keret s, Liarski V, Ascherman D, Mogahadam S, Oddis C, Aggarwal R. Serum Glutamate Dehydrogenase in Idiopathic Inflammatory Myopathy Patients [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/serum-glutamate-dehydrogenase-in-idiopathic-inflammatory-myopathy-patients/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-glutamate-dehydrogenase-in-idiopathic-inflammatory-myopathy-patients/