Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Reliable objective assessment of remission is important for the optimal management of rheumatoid arthritis (RA) patients. Calprotectin is a heterocomplex of the S100 proteins (S100A8 and S100A9), also known as MRP8/14, has been shown to correlate with RA activity. The aim of this study is to assess the use of serum calprotectin as a biomarker to differentiate between low disease activity states including remission.
Methods: The REMIRA cohort studied RA patients on > 6 months stable therapy in stable low disease activity (DAS28-ESR ≤ 3.2). They were assessed every 3 months for 1 year. Baseline, intermittent (IR) and sustained (SR) remission were defined by DAS28-ESR, DAS28-CRP, simple disease activity index (SDAI), clinical disease activity index (CDAI) and ACR/EULAR Boolean criteria. Patients not fulfilling any remission criteria at baseline were classified as ‘low disease activity state’ (LDAS). Patients not fulfilling any remission criteria over 1 year were classified as ‘persistent disease activity’ (PDA). Calprotectin levels were measured at baseline, 3, 6, 9 and 12 months. Mann-Whitney test was used for analyses of the baseline levels. For the average levels (time-integrated, ti) over 12 months, the the Jonckheere-Terpstra trend test was used. Calprotectin was measured by Crescendo Bioscience using the Buhlmann (MRP 8/14 ELISA product code EK-MRP8/14) assays.
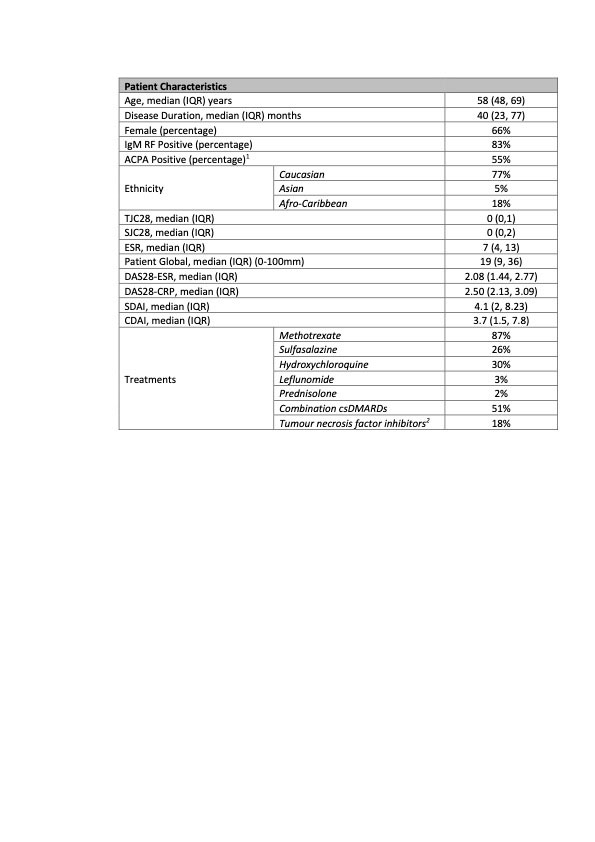
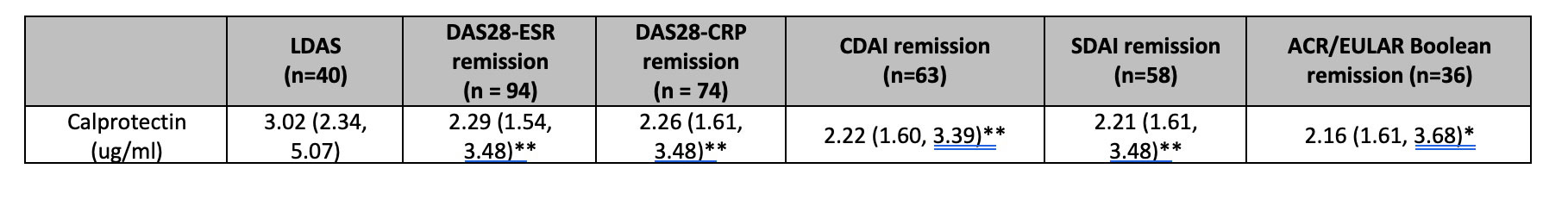
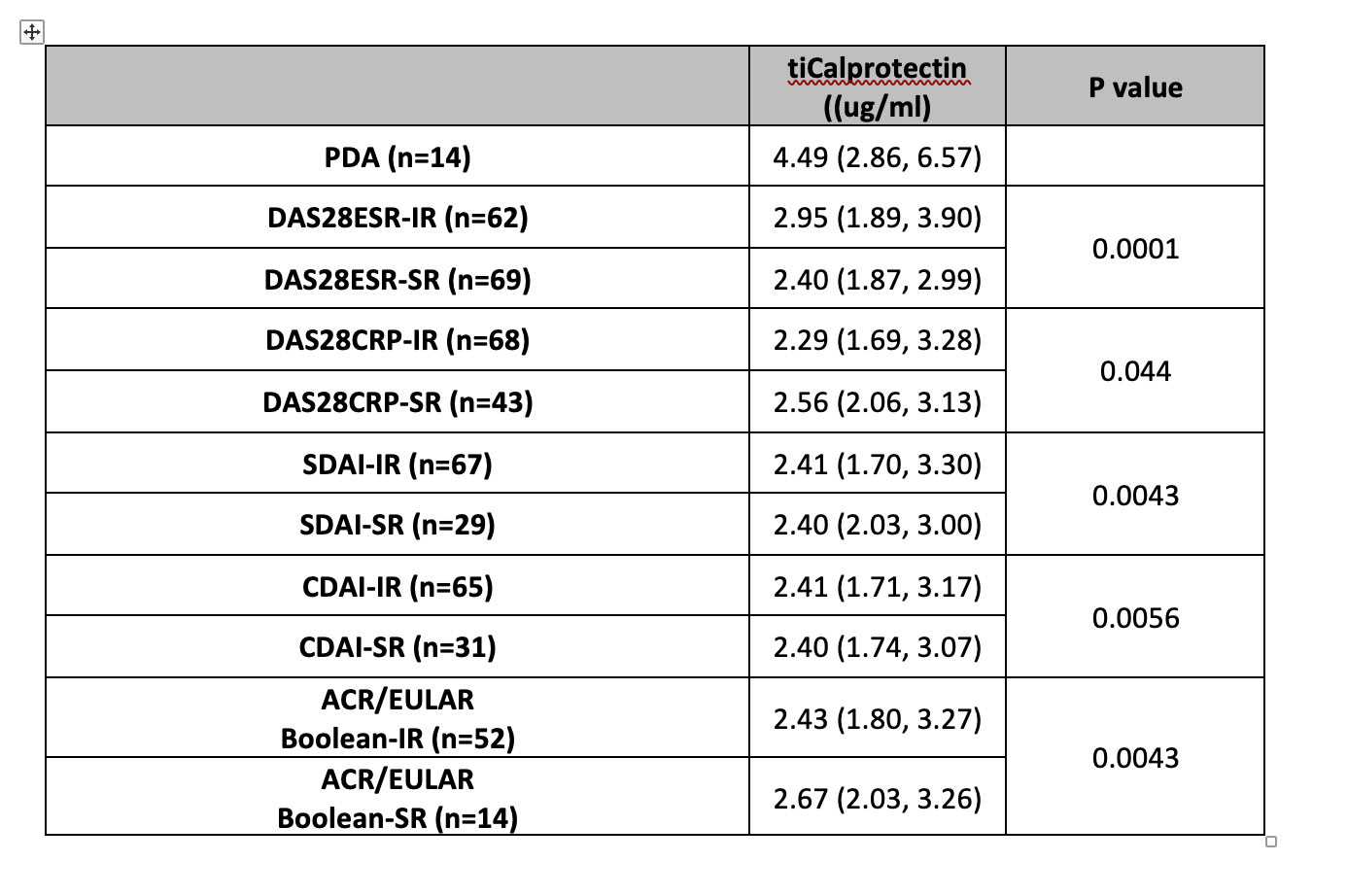
Results: Serum samples were available for 148 patients from the REMIRA cohort. Table 1 shows the patient characteristics. The median age (IQR) was 58 years (48-69), median disease duration 40 months (23-77) and 66% were female. 83% were IgM RF-positive and 55% were ACPA-positive. Of 148 patients, 28% were in the LDAS, 63% DAS28-ESR remission, 50% DAS28-CRP remission, 40% SDAI remission, 43% CDAI remission and 24% ACR/EULAR Boolean remission at baseline. Over 1 year, 9% of patients were classified as PDA. IR and SR were achieved in 42%/47% by DAS28-ESR, 46%/29% by DAS28-CRP, 45%/20% by SDAI, 44%/21% by CDAI and 35%/9% by ACR/EULAR Boolean criteria, respectively. Baseline calprotectin levels were significantly lower in all baseline remission criteria groups vs LDAS (table 2). The time-integrated values were lower among patients who achieved IR/SR vs PDA over 1 year (table 3).
Conclusion: This study demonstrated the role of calprotectin in differentiating subtle low disease activity states. Serum calprotectin was significantly different between low disease activity and remission as defined using different remission criteria. Its utility was seen with point remission as well as longitudinally over 1 year.
Comparison of the low disease activity state (LDAS: defined as not fulfilling any clinical remission criteria) group to the DAS28-ESR, DAS28CRP, CDAI, SDAI and Boolean remission groups at baseline. LDAS was defined as patients who were not in remission by any definition at baseline.
Values are median (IQR). Levels of significance determined by Mann Whitney test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Comparison of baseline values in the persistent disease activity (PDA, i.e., no remission by any criteria at any visit) group, the intermittent remission (IR) group and the sustained remission (SR) group, based on DAS28-ESR, DAS28-CRP, SDAI, CDAI and ACR/EULAR Boolean definitions of remission. Values are median (IQR). Values across the PDA/IR/SR groups were assessed using Jonckheere-Terpstra trend test.
To cite this abstract in AMA style:
Ma M, Ibrahim F, Scott D, Cope A. Serum Calprotectin Can Differentiate Between Sustained Remission and Low Disease Activity States in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/serum-calprotectin-can-differentiate-between-sustained-remission-and-low-disease-activity-states-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-calprotectin-can-differentiate-between-sustained-remission-and-low-disease-activity-states-in-rheumatoid-arthritis/