Session Information
Date: Monday, November 14, 2022
Title: Abstracts: Epidemiology and Public Health II: COVID, Infection and Pregnancy Outcomes
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: During pregnancy, best practice guidelines suggest discontinuing tumour necrosis factor inhibitors (TNFi) with high placental transfer before or during the third trimester if the maternal disease is well controlled. This recommendation stems from concerns that TNFi, which can cross the placenta, could cause immunosuppression in the offspring, particularly in the third trimester, when the placental transfer of maternal IgG is highest. However, to date, there is limited evidence on the risk of serious infections by TNFi subtypes, particularly following TNFi administration during late pregnancy. We herein evaluated the risk of serious infections in offspring born to mothers with chronic inflammatory diseases who used TNFi with high versus low placental transfer during late pregnancy (i.e., within 12 weeks prior to delivery).
Methods: In this retrospective cohort study, we identified singleton offspring born alive between 2011 and 2019 to women with a prior diagnosis of rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis, and/or inflammatory bowel disease (IBD) in the IBM MarketScan commercial claims database. TNFi exposure was defined as ≥1 filled prescription and/or infusion procedure code within 12 weeks prior to delivery. TNFi exposure was further categorized into high (i.e. infliximab, adalimumab, golimumab) and low (i.e. certolizumab, etanercept) placental transfer ability. Serious infections were ascertained based on ≥1 hospitalization with infection as the primary diagnosis in the offspring’s first year of life. We performed multivariable time-to-event analysis using a Cox proportional hazards model, adjusting for maternal age at delivery, chronic inflammatory disease diagnosis, maternal co-morbidities (pre-gestational diabetes, asthma), pregnancy complications (gestational diabetes, preterm birth), and concomitant drug use (corticosteroids and non-biologic DMARDs).
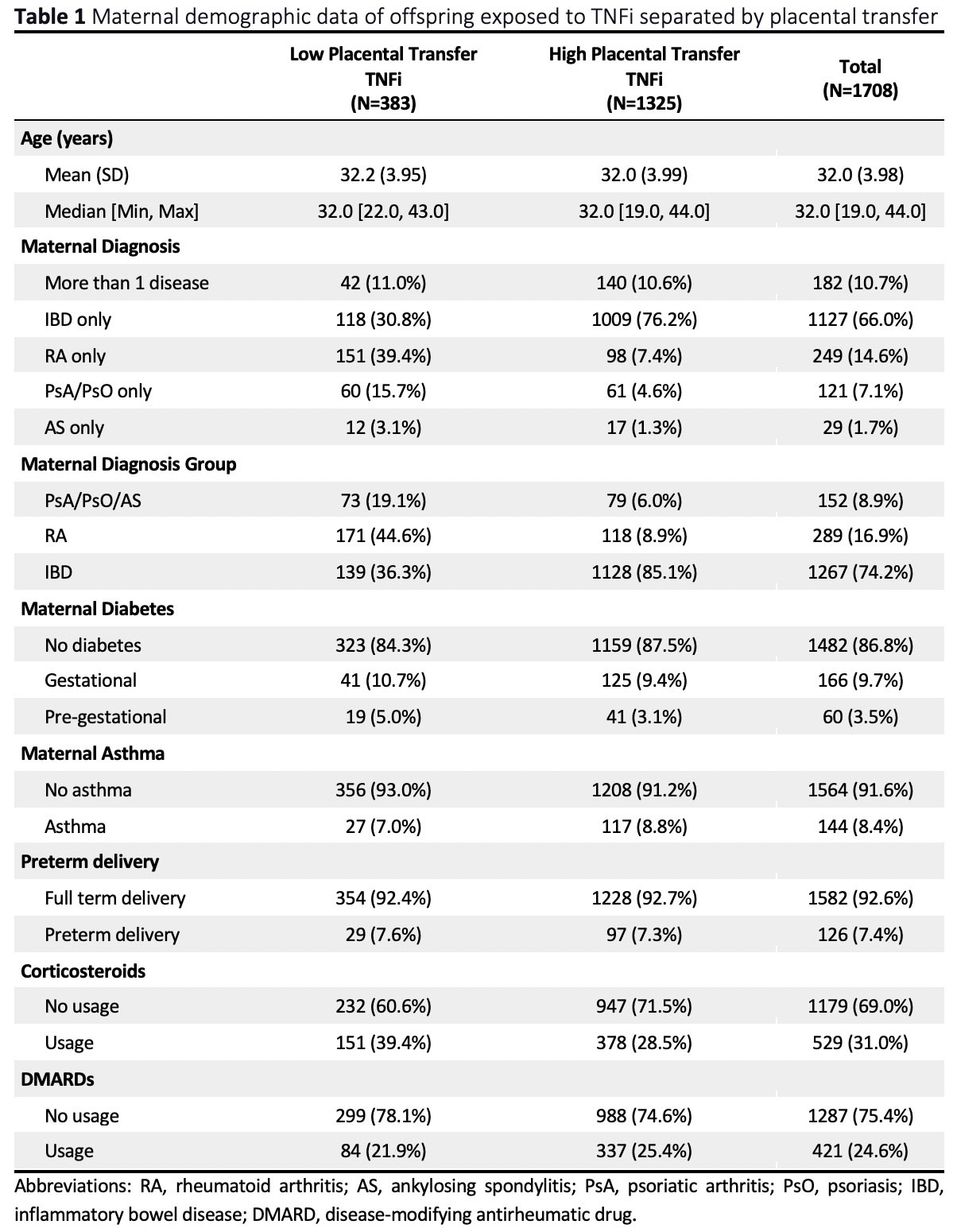
Results: We identified 26,088 offspring, among whom 1,708 (6.5%) were exposed to TNFi within 12 weeks prior to delivery. The majority of TNFi-exposed children (1,127; 66.0%) were born to mothers with only IBD (Table 1). Of the 1,708 with TNFi exposure, 1,325 (77.6%) and 383 (22.4%) had high and low placental transfer drugs, respectively. The frequency of serious infections was 2.1% in offspring exposed to TNFi with high placental transfer versus 1.6% with low placental transfer. In multivariable analyses of TNFi exposures within 12 weeks of delivery, the adjusted hazard ratio for serious infections comparing high versus low placental transfer TNFi was 0.98 (95% CI 0.36, 2.61) (unadjusted 1.32; 95% CI 0.54, 3.18).
Conclusion: In this large population sample reflecting real-world utilization of TNFi, we were unable to identify a clear risk of serious infections in children exposed during late pregnancy to TNFi with high versus low placental transfer, although the confidence interval around our estimate was wide.
To cite this abstract in AMA style:
Flatman L, St. Pierre Y, Malhamé I, Basso O, Berard A, Bernatsky S, Vinet E. Serious Infections in Offspring Exposed During Late Pregnancy to Tumour Necrosis Factor Inhibitors with High versus Low Placental Transfer Ability [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/serious-infections-in-offspring-exposed-during-late-pregnancy-to-tumour-necrosis-factor-inhibitors-with-high-versus-low-placental-transfer-ability/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serious-infections-in-offspring-exposed-during-late-pregnancy-to-tumour-necrosis-factor-inhibitors-with-high-versus-low-placental-transfer-ability/