Session Information
Date: Saturday, November 6, 2021
Title: Sjögren's Syndrome – Basic & Clinical Science Poster (0296–0322)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Primary Sjögren’s syndrome (pSS) is characterized by B-cell hyperactivity and elevated serum and saliva B-lymphocyte stimulator (BLyS) levels.1 Sequential administration of belimumab (BEL; anti-BLyS) and rituximab (RTX; anti-CD20) is a promising strategy to target B cells through distinct but complementary mechanisms.2 This analysis assesses the changes in minor salivary gland (MSG) and peripheral blood B cells following sequential administration of subcutaneous (SC) BEL and intravenous (IV) RTX (BEL/RTX) in patients with pSS.
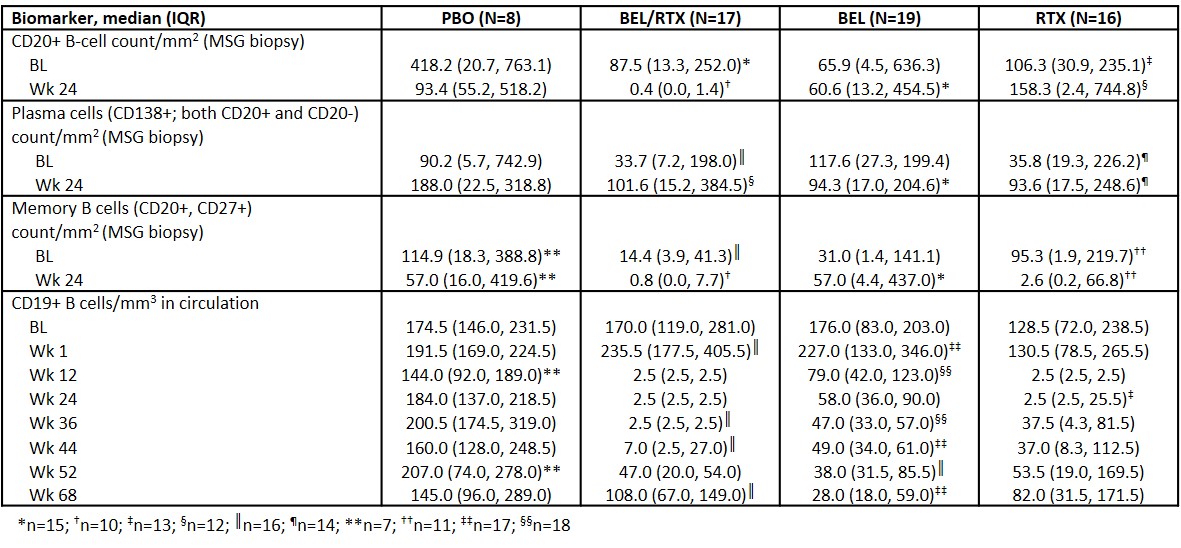
Methods: This Phase 2, double-blind study (GSK Study 201842; NCT02631538) randomized 86 adults with active pSS to placebo (PBO; N=13), BEL/RTX (N=24; BEL 200 mg SC weekly to Week [Wk] 24 followed by PBO SC weekly to Wk 52 plus RTX 1000 mg IV, Wk 8 + 10), BEL monotherapy (N=24; BEL 200 mg SC weekly to Wk 52), or RTX monotherapy (N=25; RTX 1000 mg IV, Wk 8 + 10). Primary and secondary endpoints were safety and efficacy.3 Reported here are the exploratory biomarker endpoints, performed on the completer population (N=60; patients who completed the treatment phase and follow-up). The analyses include CD20+ B-cell, plasma cell and memory B-cell quantification within MSG biopsies (baseline + Wk 24), and changes in peripheral blood B-cell numbers over time to Wk 68.
Results: MSG histology demonstrated that the numbers of infiltrating CD20+ B cells at Wk 24 were lowest in the BEL/RTX arm, with incomplete depletion observed in the monotherapy and PBO arms (Table). Although the levels of MSG memory B cells (CD20+, CD27+) were also lowest in the BEL/RTX and RTX arms, plasma cell numbers were maintained irrespective of treatment. Total peripheral blood CD19+ B cells in circulation were almost completely depleted in the BEL/RTX and RTX arms (Table). In the BEL/RTX arm, after BEL discontinuation at Wk 24, a trend of delayed repopulation of total B cells in circulation was evident relative to RTX. In the BEL/RTX and BEL arms, circulating memory B cells increased initially (baseline [BL]/Wk 1 median [interquartile range, IQR] number of cells/mm3: BEL/RTX: 28.0 [13.0, 36.0]/47.0 [30.0, 72.5], BEL: 18.0 [14.0, 30.0]/45.0 [19.0, 63.0]), and then returned toward baseline levels over time. However, in the BEL/RTX arm, following the BEL-induced increase, circulating memory B cells reached near complete depletion (Wk 24 median [IQR]: BEL/RTX: 0.2 [0.1, 0.4] cells/mm3) and remained supressed until Wk 68 (median [IQR]: 3.5 [1.5, 5.0] cells/mm3).
Conclusion: Relative to BEL or RTX monotherapy, BEL/RTX showed trends for near complete depletion of B cells in MSG, greater depletion of circulating memory B cells, and a longer period of sustained B-cell depletion. Overall, these data support the hypothesis that combined anti-BLyS and anti-CD20 act in a mechanistically complementary manner in pSS. This sequential therapy represents a novel treatment option for patients with pSS with moderate-to-severe disease activity.
Funding: GSK
References:
1Lavie F, et al. Scand J Immunol 2008;67:185–92
2Teng YKO, et al. BMJ Open 2019;9:e025687
3Mariette X, et al. Ann Rheum Dis 2021;80(Suppl 1):78–9
To cite this abstract in AMA style:
van Maurik A, Gardner D, Nayar S, Smith C, Clark K, Mistry P, Punwaney R, Roth D, Henderson R, Mariette X, Barone F. Sequential Administration of Belimumab and Rituximab in Primary Sjögren’s Syndrome Reduces Minor Salivary Gland–Resident B Cells and Delays B-Cell Repopulation in Circulation [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/sequential-administration-of-belimumab-and-rituximab-in-primary-sjogrens-syndrome-reduces-minor-salivary-gland-resident-b-cells-and-delays-b-cell-repopulation-in-circulation/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sequential-administration-of-belimumab-and-rituximab-in-primary-sjogrens-syndrome-reduces-minor-salivary-gland-resident-b-cells-and-delays-b-cell-repopulation-in-circulation/