Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Assessment and monitoring of disease activity and functioning is of major importance for qualified management of patients with axial spondyloarthritis (axSpA). This includes tight control strategies with strict monitoring and adaptation of therapy. Although closer monitoring is superior to routine care, more intensive treatment schedules can often not be realized because of time constraints and shortage of personal resources. Using a health app for the recording of patient reported outcomes (PRO) in clinical routine may facilitate patient monitoring but there is a lack of data on this strategy.
The aim of this study was to investigate the practical use and the adherence to a commercialised health app by axSpA patients with respect to usability, feasibility, and equivalence of data in clinical management.
Methods: Consecutively included patients with a clinical diagnosis of axSpA were asked to submit electronic patient-reported outcomes (ePROs) such as pain and BASDAI scores regularly but at least every 2 weeks over a period of 6 months. All patients were instructed to handle the free of charge AxSpA Live App, a Class I certified medical device, that was available for Android and iOS operating systems. In addition to patient and disease characteristics, information on previous experience with health apps was collected. The first clinical visit was followed by two face-to-face visits, each 3 months apart, in which patients completed BASFI, pain (NRS 0-10), ASDAS, and the Mobile App Rating Scale (MARS) and the System Usability Scale (SUS) to assess the quality and usability of the app.
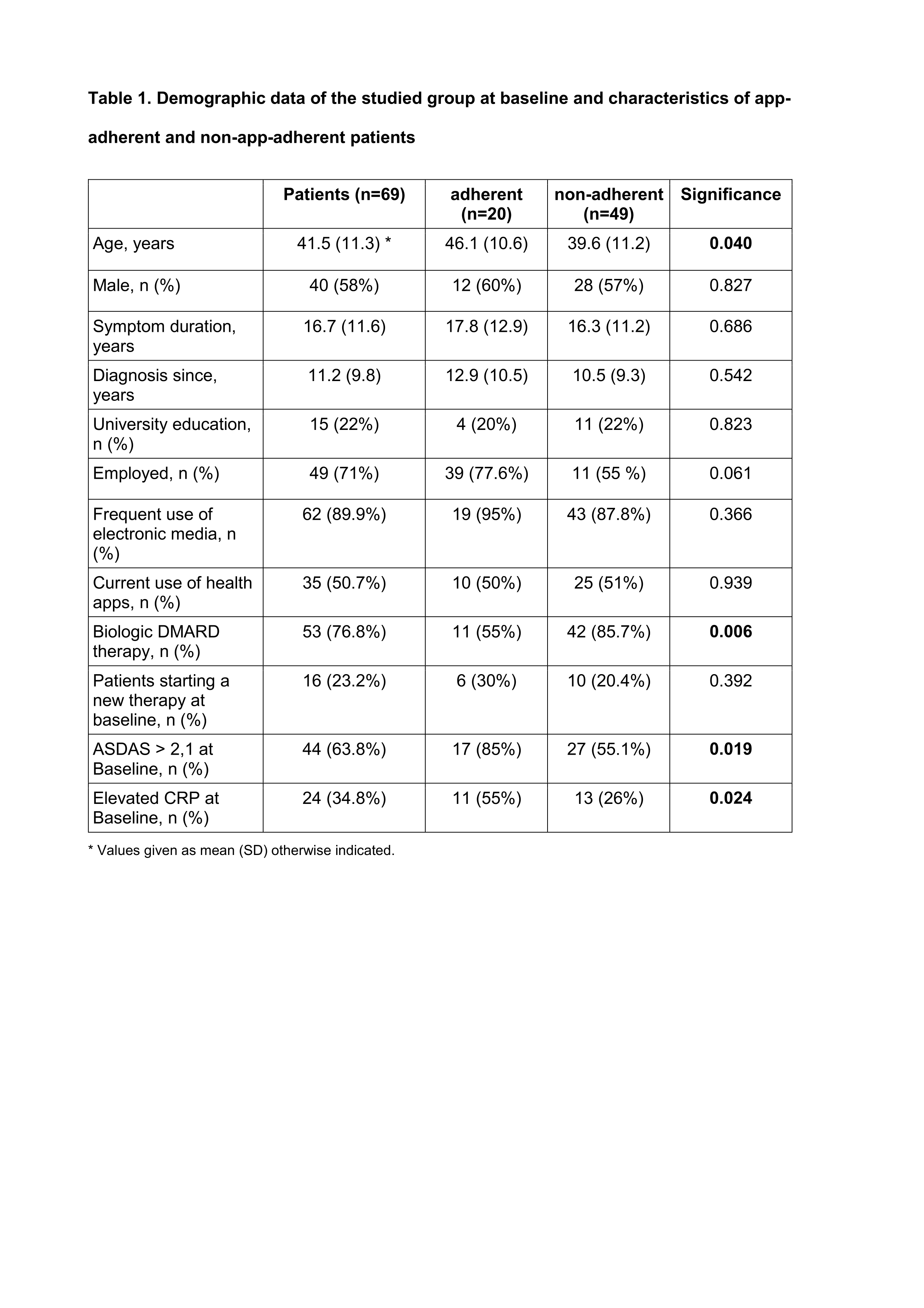
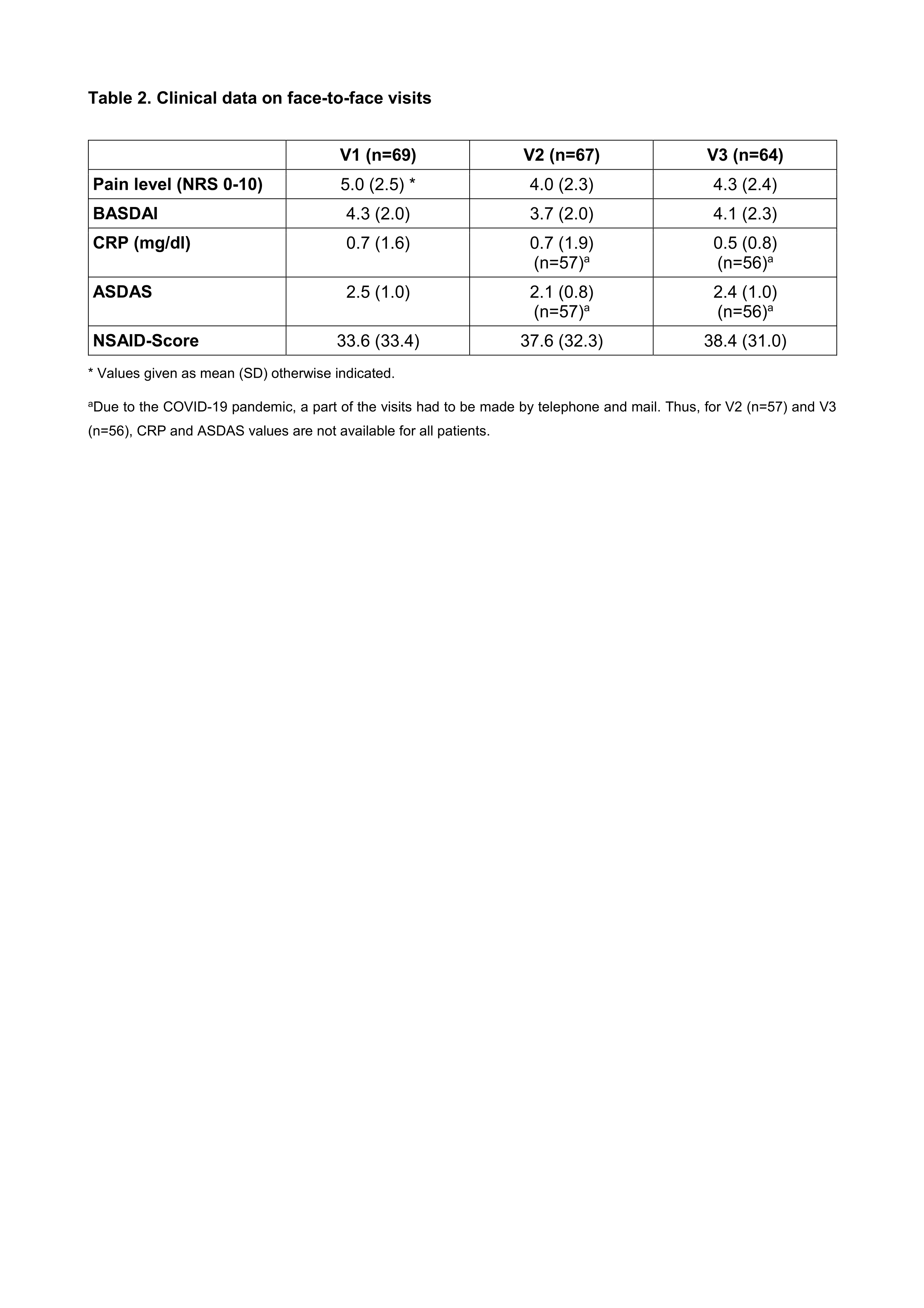
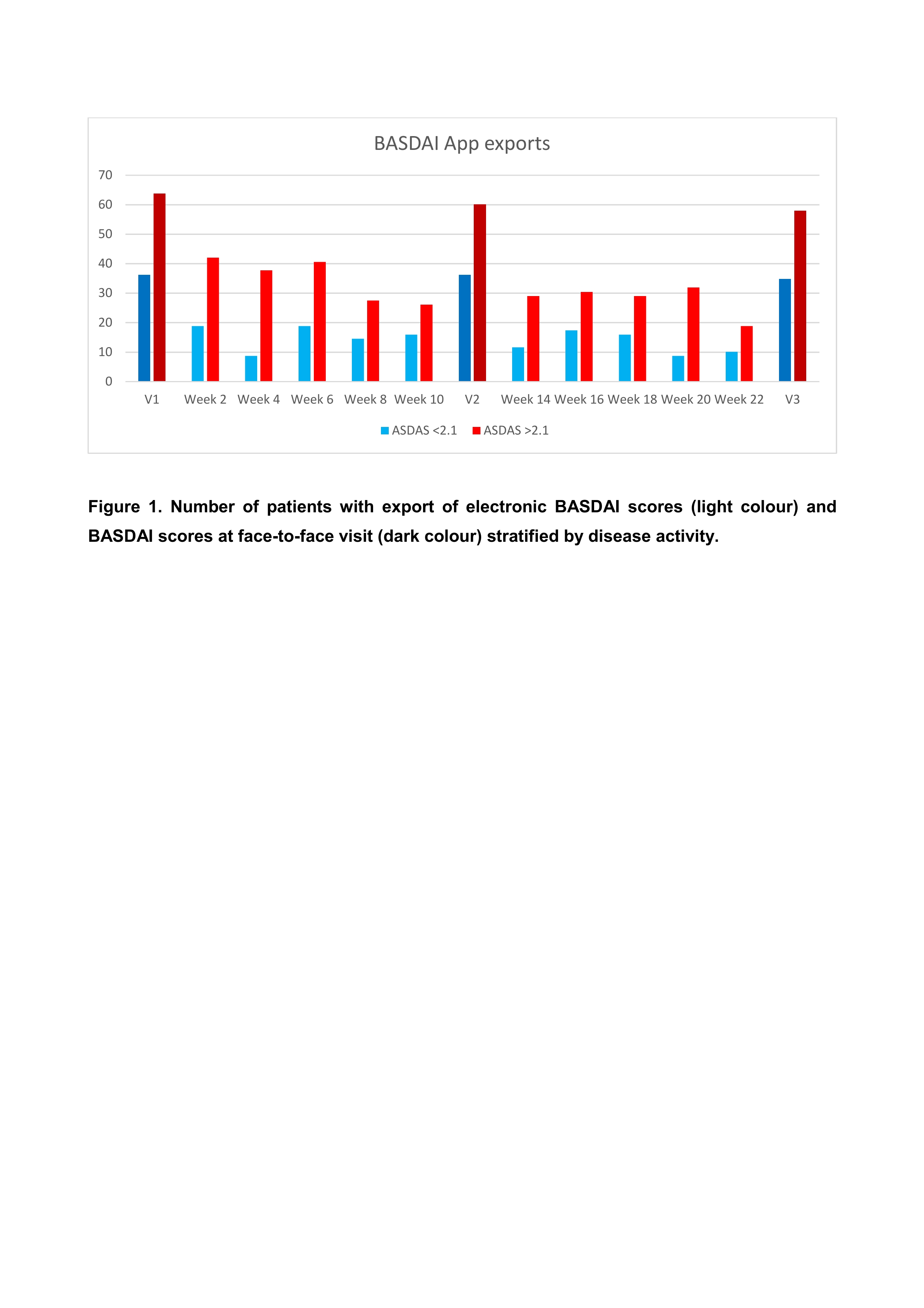
Results: Out of 103 axSpA patients asked, 69 agreed to use the AxSpA Live App (67%) on a regular basis. Five patients did not have a smartphone, one was unable to download the app for technical reasons, and 28 had other personal reasons (Tables 1 and 2). Among the participating patients 62 reported to use electronic media frequently (89.9%), and they had previously used health apps (mean number of apps used 1.0 ± 1.3). The majority (64 (92.8%)) of patients completed 6 months (92.8%). Patients’ adherence for app transmissions every 2 weeks was low, with 29% and 28.6% after 3 and 6 months, respectively. Significant predictors for a good adherence were high disease activity as assessed by ASDAS (P = 0.019) and older age (P = 0.04). There were no systematic differences between BASDAI scores documented on paper or by app (ICC 0.99 (95%CI 0.98 – 0.99). The quality and usability of the app was rated with a mean MARS and SUS scores of 3.6 and 71.2, respectively, which corresponds to a good to acceptable rating.
Conclusion: The majority of patients with axSpA was able to use the AxSpA Live App but adherence over 6 months was poor. Poor adherence rate can possibly be explained by the high number of required transfers. High disease activity and older age had a positive influence on the frequency of reporting. Our data suggest that the use of apps should focus on more severely affected patients. Interventions with use of a digital application seems feasible for axSpA patients with high disease activity who are in need for intensified treatment.
Table legend on the bottom: * Values given as mean (SD) otherwise indicated.
Table legend on the bottom:
* Values given as mean (SD) otherwise indicated.
a Due to the COVID_19 pandemic, a part of the visits had to be made by telephone and mail. Thus, for V2 (n=57) and V3 (n=56), CRP and ASDAS values are not available for all patients.
To cite this abstract in AMA style:
Kiltz U, Kempin R, Richter J, Schlegel A, Baraliakos X, Tsiami S, Buehring B, Kiefer D, Braun J. Self-monitoring of Disease Activity with a Smartphone App Is Feasible in Routine Clinical Management of Patients with Axial Spondyloarthritis – a Proof of Concept Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/self-monitoring-of-disease-activity-with-a-smartphone-app-is-feasible-in-routine-clinical-management-of-patients-with-axial-spondyloarthritis-a-proof-of-concept-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/self-monitoring-of-disease-activity-with-a-smartphone-app-is-feasible-in-routine-clinical-management-of-patients-with-axial-spondyloarthritis-a-proof-of-concept-study/