Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Secukinumab (SEC), a fully human monoclonal antibody selectively neutralizing interleukin-17A, exhibits significant efficacy, with a favorable safety profile, in the treatment of psoriatic arthritis (PsA) and moderate to severe psoriasis. SEC has a rapid onset of action and demonstrates sustained responses. PsA and psoriasis patients are at greater risk for psychological distress, including depression and suicidality. Previous analysis, using pooled data from phase 3 studies FIXTURE and ERASURE, reported high and sustained relief from anxiety/depression in psoriasis patients treated with SEC 300 mg up to Week (Wk) 52 (~80% of patients reported not being anxious/depressed) from the EuroQol 5-dimensional (Mobility, Self-Care, Usual Activities, Pain/Discomfort, and Anxiety/Depression) and 3-level questionnaire (EQ-5D-3L). Here, we aim to confirm these results in additional phase 3 SEC trials in patients with PsA and psoriasis.
Methods: Results of the ‘Anxiety/Depression’ dimension ("not anxious/depressed", "moderately anxious/depressed", or "extremely anxious/depressed) of EQ-5D-3L were derived from three phase 3 studies using an approved SEC dose: FUTURE 1 and FUTURE 2, two randomized, double-blind clinical trials, comparing SEC 150 mg to placebo in active PsA; and CLEAR, a multicenter, double-blind, parallel-group study comparing SEC 300 mg to ustekinumab in moderate to severe psoriasis. The proportions of patients reporting as not being anxious/depressed are described as observed for each individual study up to Wk 52.
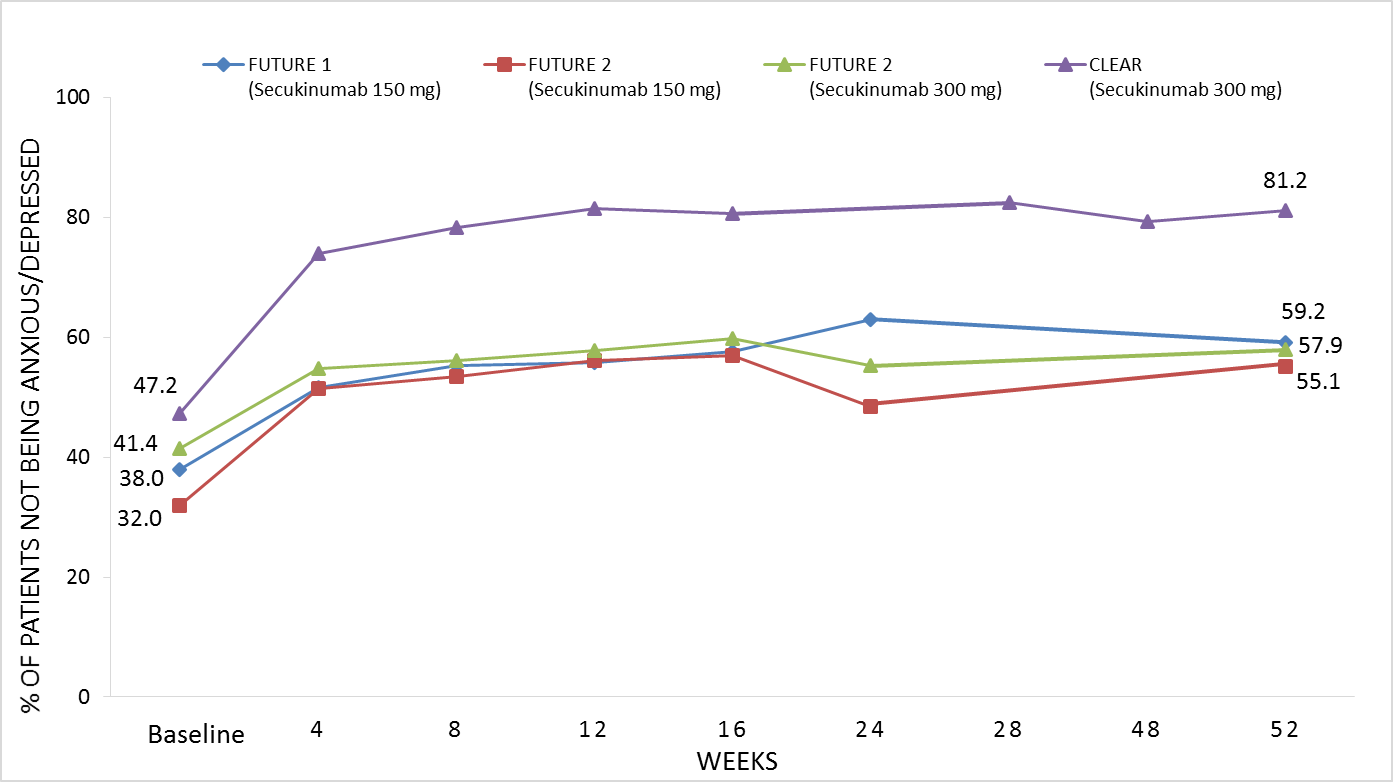
Results: In FUTURE 1, 76/200 (38.0%) of PsA patients treated with SEC 150 mg reported being not anxious/depressed at baseline, increasing to 99/192 (51.6%) at Wk 4 and 109/184 (59.2%) at Wk 52 (Fig. 1). Similarly, in FUTURE 2, 32/100 (32.0%) and 41/99 (41.4%) of PsA patients treated with SEC 150 mg or 300 mg, respectively reported being not anxious/depressed at baseline, increasing to 51/99 (51.5%) and 52/95 ( 54.7%) at Wk 4 and 49/89 (55.1%) and 55/95 (57.9%) at Wk 52 (Fig. 1). In the CLEAR study, 154/326 (47.2%) psoriasis patients treated SEC 300 mg reported being not anxious/depressed at baseline, and this increased to 238/322 (73.9%) at Wk 4, 263/326 (80.7%) at Wk 16, and was sustained up to Wk 52 (237/292, 81.2%) (Fig. 1).
Conclusion: This analysis of the patient-reported EQ-5D-3L anxiety/depression measure from three phase 3 SEC trials showed consistently higher anxiety/depression burden among patients with PsA than among those with moderate to severe psoriasis; however, it also indicates that SEC treatment improves and provides sustained relief from anxiety/depression among all treated patients, regardless of their disease, up to 1 year.
Figure 1. Percentages of patients with psoriatic arthritis or psoriasis reporting not being anxious/depressed on EQ-5D following treatment with secukinumab in three phase 3 clinical trials (as observed).
To cite this abstract in AMA style:
Mease PJ, Lebwohl M, Gilloteau I, Fox T, Oliver J, Jugl S, Gottlieb AB. Secukinumab Treatment of Psoriatic Arthritis and Moderate to Severe Psoriasis Relieves Anxiety/Depression up to 52 Weeks: An Overview from Secukinumab Phase 3 Clinical Trials [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/secukinumab-treatment-of-psoriatic-arthritis-and-moderate-to-severe-psoriasis-relieves-anxietydepression-up-to-52-weeks-an-overview-from-secukinumab-phase-3-clinical-trials/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-treatment-of-psoriatic-arthritis-and-moderate-to-severe-psoriasis-relieves-anxietydepression-up-to-52-weeks-an-overview-from-secukinumab-phase-3-clinical-trials/