Session Information
Date: Sunday, November 17, 2024
Title: Systemic Sclerosis & Related Disorders – Basic Science Poster I
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Investigation of Transcriptional Changes in Interstitial Lung Disease (ILD) and Exploration of Functional Effects of Differential Genes and Expressed Proteins in the Development of ILD.
Methods: This study analyzed ILD patients’ lung tissue transcriptomic datasets using bioinformatics to identify differentially expressed genes. An ILD mouse model was created with bleomycin, and mRNA sequencing compared gene transcription levels between early/late-stage ILD mice and controls. Cross-analysis of human and mouse data identified common genes, with their functions and pathways further analyzed. The expression of SFRP2 in lung tissue, peripheral blood and ILD mice of CTD-ILD patients was detected by multiple techniques. An in vitro EMT model was established using TGF-β1-stimulated A549 cells, with recombinant sFRP2 to assess EMT and Wnt signaling markers, while Wnt inhibitor IWR-1 was used to explore sFRP2-induced EMT mechanisms. Immunofluorescence evaluated EMT-related proteins and sFRP2 in CTD-ILD patients and ILD mice.
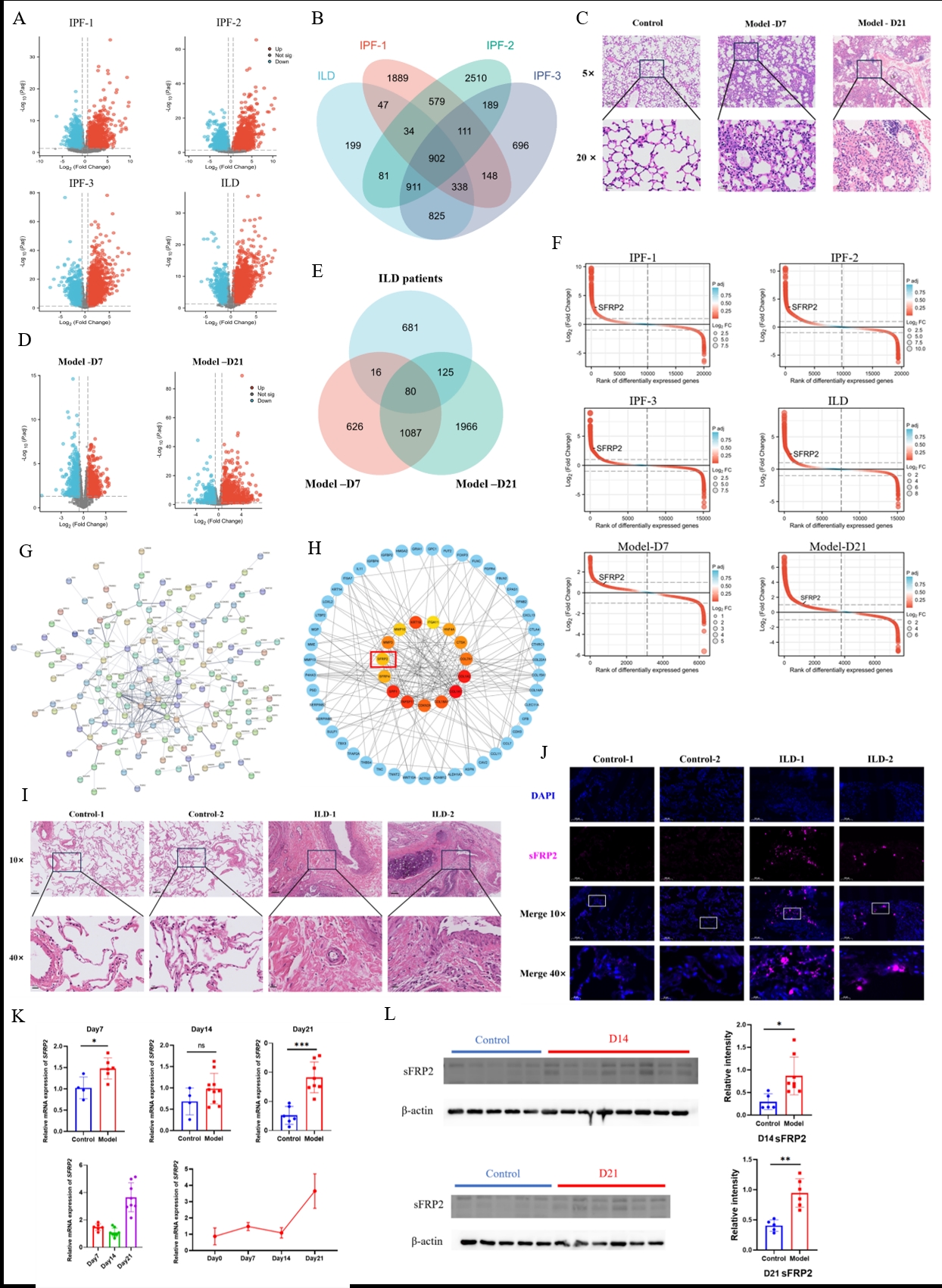
Results: 1. Transcriptomic analysis of lung tissues from four ILD patient groups identified 4048, 5317, 4120, and 3337 differentially expressed genes (DEGs) respectively, with 902 showing consistent trends. In ILD model mice, 1810 and 3259 genes were significantly different at early (day 7) and late (day 21) stages compared to controls. Cross-species analysis found 80 consistent DEGs, with SFRP2 notably upregulated, highlighting its pivotal role. Functional enrichment linked SFRP2 to EMT and Wnt signaling pathways.
2. sFRP2 protein levels were significantly higher in CTD-ILD patient blood compared to controls and CTD patients without ILD. In ILD model mice, sFRP2 expression increased significantly by day 7, continued rising by day 14, and peaked by day 21. Immunofluorescence showed SFRP2 overexpression and related protein increases concentrated in alveoli and terminal bronchioles. ILD patient and model tissues also showed upregulated EMT functionality and Wnt signaling, correlating with sFRP2 expression levels.
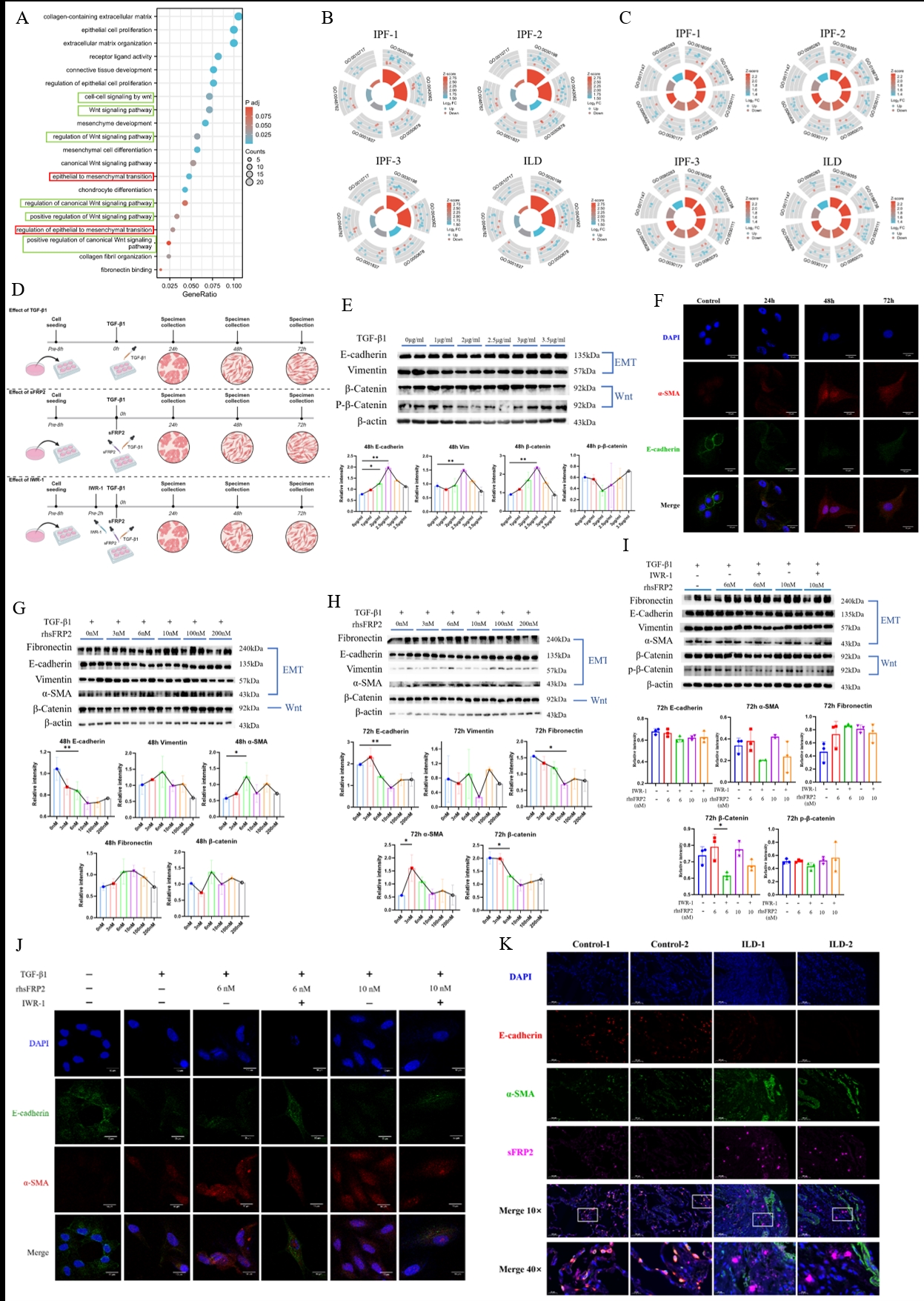
3. TGF-β1 stimulation of A549 lung epithelial cells revealed low concentrations activated the Wnt pathway, while high concentrations inhibited it. EMT and Wnt were significantly activated at 2.5 μg/ml TGF-β1, forming an in vitro EMT model. Exogenous recombinant sFRP2 at 6nM or 10nM downregulated E-cadherin, upregulated vimentin and α-smooth muscle actin, and promoted β-catenin increase, activating Wnt signaling, while higher sFRP2 concentrations (100nM, 200nM) inhibited these effects. sFRP2-induced changes were antagonized by the Wnt inhibitor IWR-1, reducing fibrosis-related fibronectin production.
Conclusion: 1. The SFRP2 gene is found to be excessively transcribed in the early stages of ILD and maintains high expression throughout the disease progression.
2. The sFRP2 protein can induce EMT in lung epithelial cells by activating the Wnt signaling pathway, thereby participating in the occurrence and development of ILD.
3. Administration of Wnt signaling pathway inhibitors can attenuate the EMT function promoted by sFRP2 protein, indicating its potential as a therapeutic target for alleviating fibrotic lesions of ILD.
Target gene SFRP2 was detected and its expression was increased by ILD transcriptome screening.
A. Transcriptomic differential genes between four ILD patients and control groups; B. Common differential genes with consistent changes in four groups of ILD patients; C. The pathological changes of lung tissue in ILD mouse model were detected by H&E staining; D. Transcriptomic differential genes of ILD model mice at day 7 and 21; E. A common differential gene in ILD model mice and ILD patients; F. The variation of SFRP2 in different transcriptome differential genes was ranked; G. Human and mouse common differential genes construct protein interaction network; H. The top 15 hub genes and their first-order interaction genes; I. The pathological results of lung tissue in CTD-ILD patients were compared with those in CTD-ILD; J. Expression of sFRP2 in lung tissue of CTD-ILD patients; K. Expression of SFRP2 gene in lung tissue of mouse model; L. Expression of sFRP2 protein in lung tissue of mouse model.
Mechanism and pathway exploration of SFRP2 in ILD.
A. Functional enrichment of common differential genes in mice and human and correlation results of SFRP2 gene; B. Regulatory trends of EMT-related pathways in lung tissue of ILD patients; C. Regulatory trend of WNT-related pathways in lung tissue of ILD patients; D. Schematic diagram of cell experimental treatment in vitro; E. Expression of EMT and WNT-related proteins after 48h stimulation with TGF-β1 at different concentrations; F. The EMT-associated proteins of A549 stimulated by 2.5μg/ml TGF-β1 at different time points were detected by immunofluorescence; G. Effects of different concentrations of rhsFRP2 treated for 48h on the expression of related proteins in EMT model in vitro; H. Effects of different concentrations of rhsFRP2 treated for 72h on the expression of related proteins in EMT model in vitro; I. Effect of IWR_1 treatment for 72h on expression of RHSFRP2-stimulated EMT in vitro; J. EMT-related proteins were expressed after 72h in each treatment group; K. Expression of sFRP2 and EMT-related proteins in lung tissue of CTD-ILD patients.
To cite this abstract in AMA style:
Wu Y, Li Y, Liu Y. Secreted Frizzled-Related Protein 2 (sFRP2) Regulates Wnt Signaling to Affect Mesenchymal Transition of Lung Epithelial Cells Participates in Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/secreted-frizzled-related-protein-2-sfrp2-regulates-wnt-signaling-to-affect-mesenchymal-transition-of-lung-epithelial-cells-participates-in-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secreted-frizzled-related-protein-2-sfrp2-regulates-wnt-signaling-to-affect-mesenchymal-transition-of-lung-epithelial-cells-participates-in-interstitial-lung-disease/