Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Currently, social determinants of health (SDoH; conditions in which people live) are not routinely screened for in US outpatient rheumatology clinics.1 SLE disproportionally affects patients from underrepresented groups and of lower socioeconomic status, with black patients and those with public insurance more likely to have fragmented care, leading to worse clinical outcomes.2 This study determined the feasibility of routine SDoH screening at the point-of-care in tertiary lupus clinics, and associated barriers and facilitators at physician, care team, and patient levels.
Methods: This observational, prospective, mixed methods study (GSK Study 219011) was conducted in two large, academic lupus clinics in the US. Patients ≥18 years with an established SLE diagnosis and an appointment at one of the clinics during the study were screened for SDoH. Study implementation was completed over 2 weeks (Institution 1, Jul–Aug 2023) and 7 weeks (Institution 2, Aug–Oct 2023). Patient-reported responses to SDoH domains chosen by participating institutions were collected. Patient demographics were collected and an optional patient experience survey was administered to understand SDoH screener readability, comprehension, and willingness to respond. Semi-structured post-implementation interviews with care team members and institutional health equity leaders took place to understand implementation, tool usability and comprehension, clinician acceptance, facilitators of use, and institutional health equity policies. Transcripts were analyzed using thematic analysis.
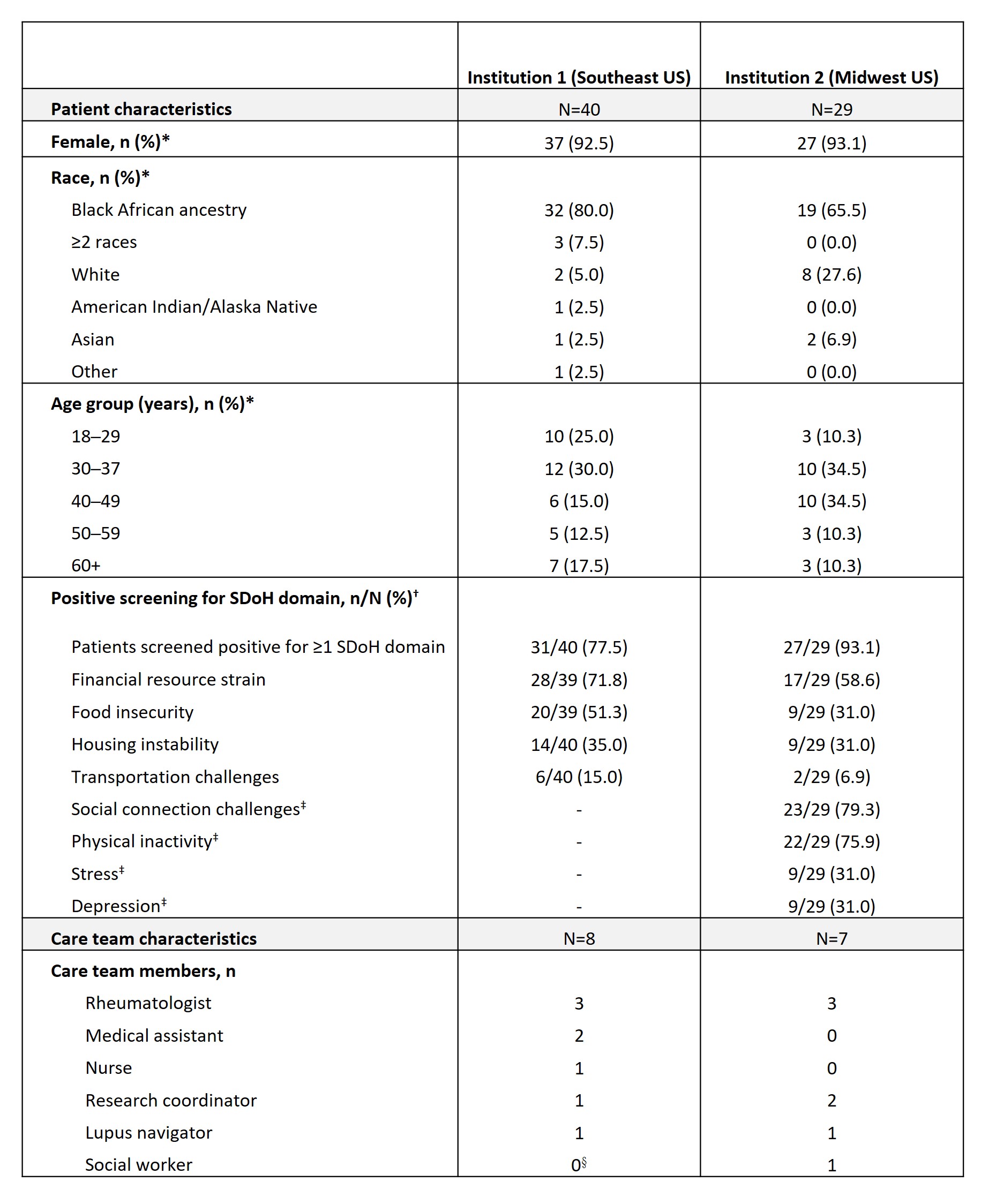
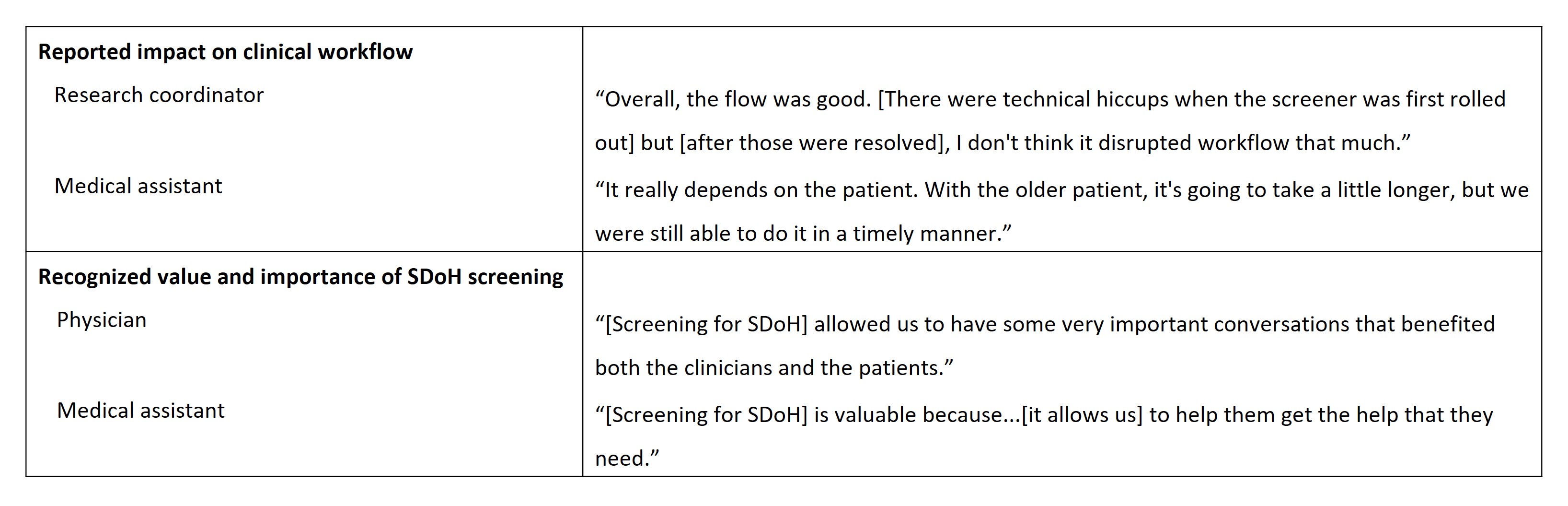
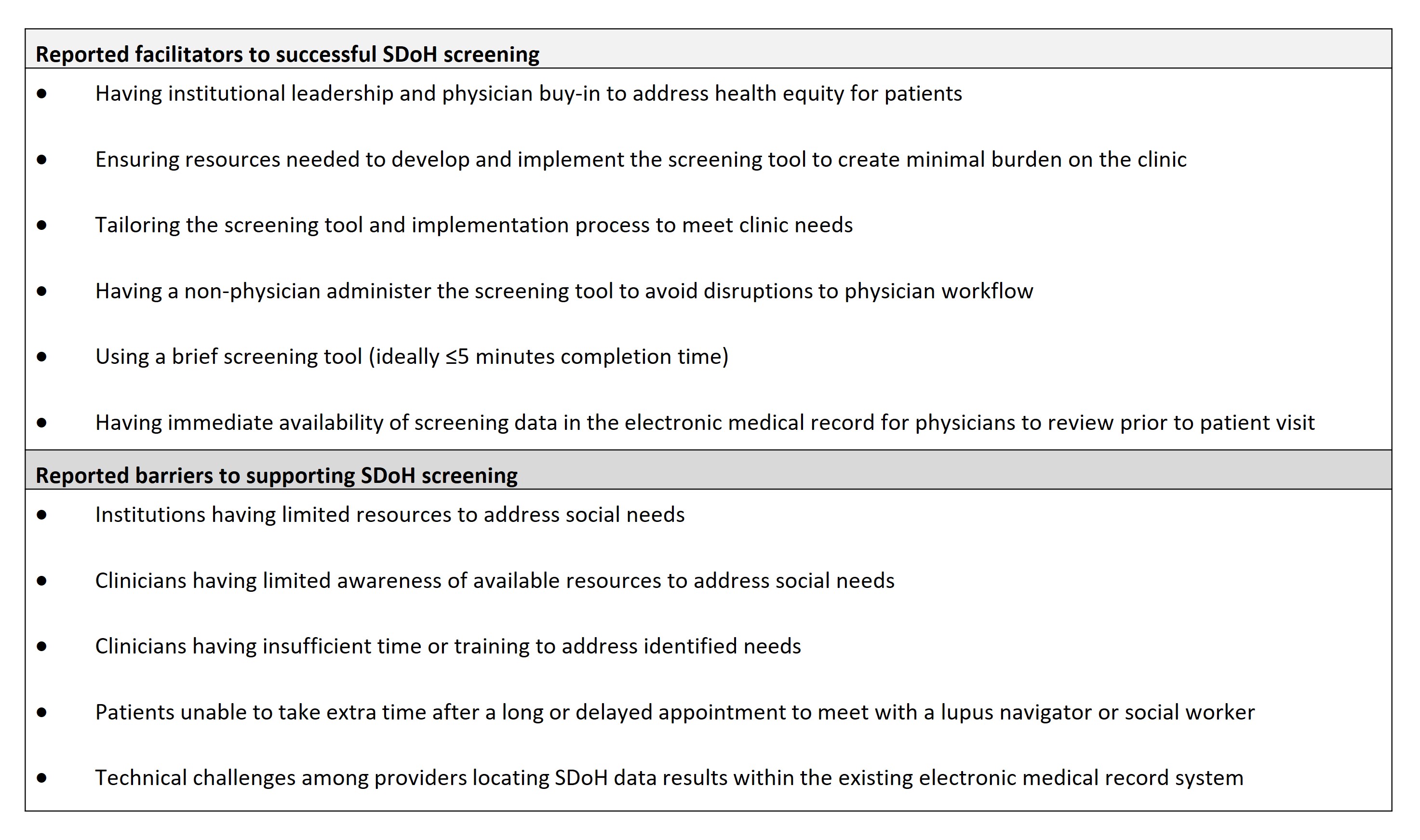
Results: In total, 69 patients with SLE participated in the routine screening of SDoH across the 2 institutions; 65 patients completed the patient experience survey. Overall, 77.5% of patients at Institution 1 screened positive for ≥1 of 4 domains and 93.1% at Institution 2 for ≥1 of 8 domains (Table 1). Most patients found the SDoH screening tool “very easy” to understand (91%, n=59/65), were “very comfortable” answering (80%, n=52/65), and were “happy to be asked” SDoH questions (75%, n=49/65). Qualitative interviews with 15 care team members (Table 1) indicated that implementation caused minimal disruption to clinical workflow and patients typically spent 3─10 minutes completing the tool (Table 2). Clinicians and care teams reported the value and importance of SDoH screening and wanted it to continue in their clinics (Table 2). Reported facilitators to successful SDoH screening included having institutional leadership buy-in to address health equity and a brief screening tool format (≤5 minutes); barriers included limited resources, time, or training to address social needs (Table 3).
Conclusion: Routine SDoH screening was seen as valuable by both patients and care teams. With an appropriately resourced and trained care team, SDoH screening can be successfully implemented to inform clinical decision making and patient care in lupus clinics. Future research should evaluate whether regular SDoH screening for patients with SLE can minimize healthcare disparities and improve care management.
Funding: GSK Study 219011
References
1Taber KA et al. ACR Open Rheumatol 2021;3:305─11
2Walunas TL et al. Arthritis Care Res 2017;69(9):1369–76
*Data obtained from study Data Collection Log completed by each institution; †for Institution 1, if a patient marked a response on the questionnaire for any of the questions in each domain that indicated a social need, they were considered as having a “positive” screen. For Institution 2, the present study used the same scoring algorithm that was used in Institution 2’s electronic health record. Responses to all SDoH domains were optional, therefore, the denominator varies due to missing responses; ‡domains that were only screened at Institution 2; §while Institution 1 had a social worker assigned to their clinic, that person did not sit within the clinic and did not engage in this project.
To cite this abstract in AMA style:
Lim S, Nadipelli V, Bruno M, Lew D, Rubin B, Dunlop-Thomas C, Mitchell K, Demetriou L, Berko J, Kim A. Screening for Social Determinants of Health in Patients with SLE: A Point-of-Care Feasibility Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/screening-for-social-determinants-of-health-in-patients-with-sle-a-point-of-care-feasibility-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/screening-for-social-determinants-of-health-in-patients-with-sle-a-point-of-care-feasibility-study/