Session Information
Date: Saturday, November 12, 2022
Title: Abstracts: Miscellaneous Rheumatic and Inflammatory Diseases I
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Patients with chronic inflammatory arthritis (CIA) are at increased risk for the development and mortality from COVID-19. Vaccinations are integral to the management of these conditions. Disease-modifying antirheumatic drugs (DMARDs) used to treat CIA have the potential to blunt the immune response and efficacy of vaccinations. There is little data on the effect of DMARDS used for CIA on the response to novel mRNA vaccines, although limited data is emerging from small studies. Guidelines on adjusting such therapy are limited by a paucity of available data. Our objectives were to compare the antibody response (ABR) to the SARS-CoV-2 mRNA vaccines in patients with CIA on treatment with either methotrexate (MTX), tumor necrosis factor inhibitors (TNFi), or both with healthy controls as well as determine the effect of interrupting therapy after vaccination in patients with CIA on the ABR to the vaccine.
Methods: 63 patients (ages 18-90) with rheumatoid or psoriatic arthritis on MTX, TNFi or both were recruited. All subjects received two doses of a mRNA COVID vaccine. Use of hydroxychloroquine (HCQ), NSAID’s, and prednisone (Pred) ≤10mg daily were allowed. Those with prior COVID infection were excluded, as determined by SARS-CoV-2 nucleocapsid assay. 26 healthy age-matched controls were obtained. IRB approval was obtained, and patients were consented to participate in the study. SARS Anti-receptor binding domain IgG antibodies were measured by electro chemiluminescent immunoassay 90-120 days post initial vaccine dose. Patients were divided into 3 groups based on therapy:
1. MTX monotherapy
2. TNFi with eternacept (ETN) or adalimumab (ADA)
3. A combination of MTX with either ETN or ADA
Each of the groups was subdivided into two categories:
1. Continued treatment uninterrupted at the time of each of the two vaccines.
2. Held treatment for two weeks after each vaccine.
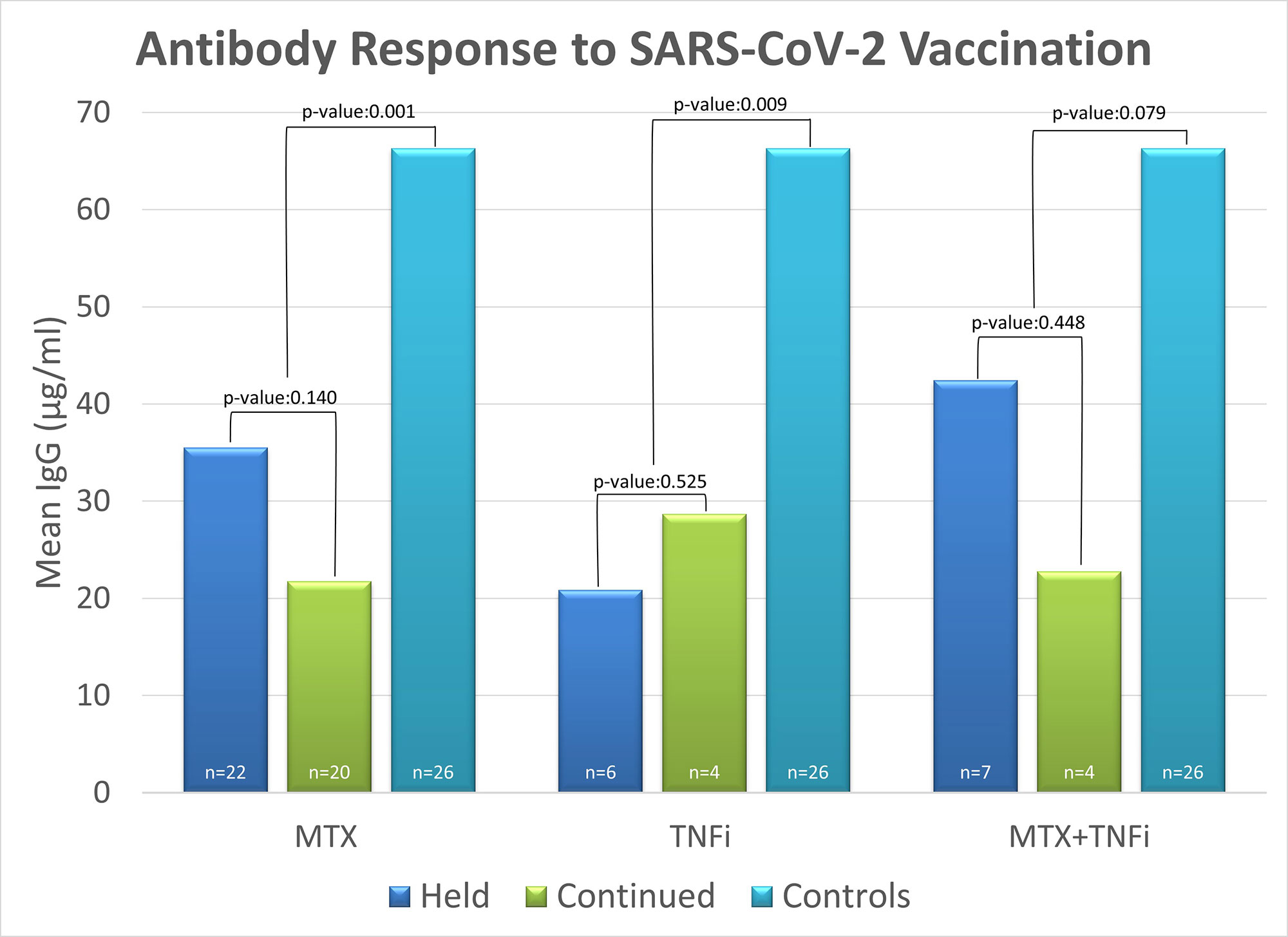
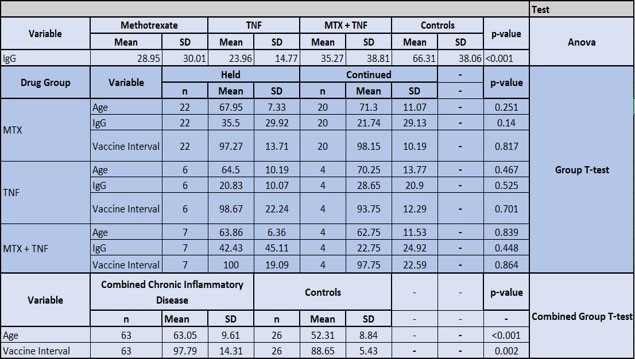
Results: The 63 patients with CIA had a significantly lower ABR to vaccine compared with healthy controls (p=0.001). Further analysis was limited by sample size: The MTX held group had a higher ABR than the MTX continued group (mean IgG=35.5 vs 21.74; p=0.14), demonstrating a trend toward increased immunogenicity. There was a similar ABR to vaccine between those on TNFi who held vs continued therapy (mean IgG 20.83 vs 28.65; p=0.525). Combination MTX +TNFi held vs continued groups demonstrated a trend toward increased immunogenicity when holding therapy post vaccine (mean IgG 42.4 vs 22.7; p=0.44). All treatment groups were comparable in Pred, HCQ, NSAID use, age, Rapid 3 score, and time interval between vaccination and blood draw for antibody levels.
Conclusion: The ABR in patients with CIA to the mRNA vaccine appeared to be blunted by ongoing therapy with MTX. This effect was attenuated by holding MTX post-vaccine. There was no difference in the ABR to vaccine in patients on TNFi who held vs continued these agents after vaccine, although the sample size was small. These findings should be validated in a larger cohort. Patients with CIA on DMARD therapy had a significantly lower ABR to the vaccine compared to healthy controls. Clinicians may consider holding MTX for two weeks post vaccination to optimize the immune response to the vaccine.
To cite this abstract in AMA style:
Darwish N, Jhaveri S, Yoganathan U, Bakillah H, Chun K, Wasser T, Freeman J. SARS-CoV-2 mRNA Vaccine Immunogenicity in Chronic Inflammatory Arthritis on DMARD Therapy [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/sars-cov-2-mrna-vaccine-immunogenicity-in-chronic-inflammatory-arthritis-on-dmard-therapy/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sars-cov-2-mrna-vaccine-immunogenicity-in-chronic-inflammatory-arthritis-on-dmard-therapy/