Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The disintegrin and metalloproteinase with thrombospondin motifs-5 (ADAMTS-5) is a key enzyme in OA (Verma P, et al. J Cell Biochem 2011;112:3507-14). In preclinical models of OA, S201086, a potent and selective inhibitor of ADAMTS-5, protects from cartilage degradation (Clement-Lacroix, et al. Osteoarthritis Cartilage 2017;25:S58). Therefore, S201086 is co-developed by Servier and Galapagos (GLPG1972) as a potential new treatment in OA.
The objective of this phase I study was to assess and compare the safety, tolerability, pharmacokinetics and pharmacodynamics of S201086 in healthy male Japanese and Caucasian subjects.
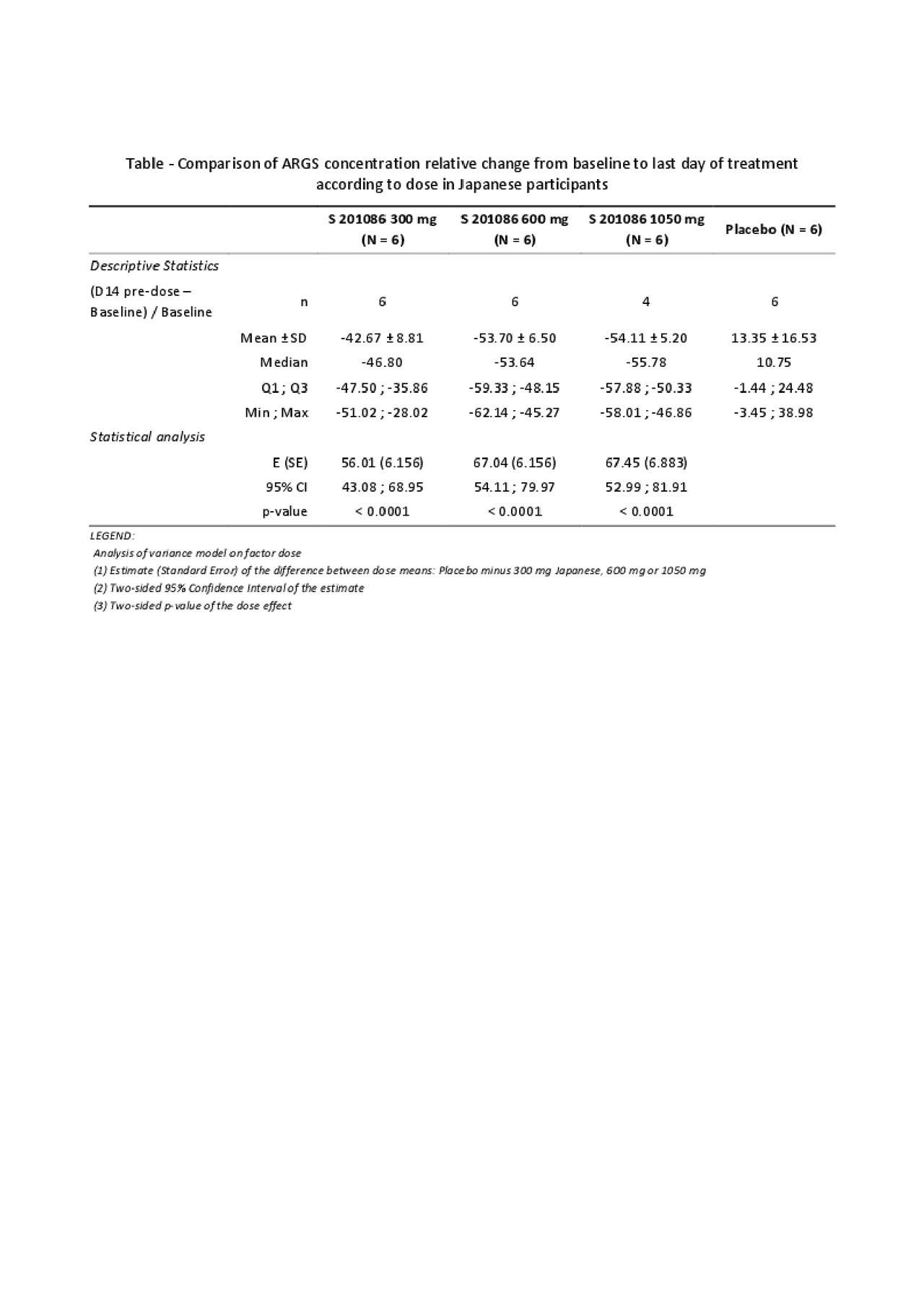
Methods: Six ascending single doses of S201086 (50 mg to 1500 mg) were administered orally as tablets in fasting conditions in 6 groups of 8 Japanese subjects (aged 20-45 years) and 3 ascending multiple doses (300, 600 or 1050 mg/day for 14 days) were administered orally as tablets in fed conditions in 3 groups of 8 Japanese subjects. Additionally, 2 cohorts of 8 Caucasian subjects received either a single oral dose of 300 mg or repeated oral dose of 300 mg once daily for 14 days. In each group, 6 subjects received S201086 and 2 placebo. Plasma was collected at several time points for the quantification of S201086 by LC-MS/MS. Plasma pharmacokinetic parameters were calculated via non-compartmental analysis using Phoenix® WinNonlin® software. Pharmacodynamics was assessed by measurement of the aggrecan ARGS neoepitope levels in serum by ELISA.
Results: No serious adverse drug reactions were observed after administration of single and multiple ascending doses of S201086 in either Japanese or Caucasian subjects. Most adverse events were of mild to moderate intensity and were resolved by the end of the study. Following single administration to Japanese subjects, Cmax and AUC increased proportionally with doses from 50 to 600 mg and slightly less than dose-proportionally from 600 to 1500 mg, consistent with what was previously observed in the first in-human study in healthy Caucasian subjects (van der Aar E, et al. Osteoarthritis Cartilage 2018;26:S310). In Japanese subjects, after 14-days administration, a limited 1.2- to 1.4-fold accumulation was observed on both Cmax and AUC at Day 14 as compared to Day 1, and the steady-state exposure increased dose-proportionally within the 300-1050 mg dose range. Taking the inter-subject variability into account, no difference was observed in Cmax and AUC between Caucasian and Japanese subjects after single administrations of 300 mg and repeated administrations of 300 mg once daily for 14 days. The serum ARGS neoepitope levels decreased between baseline and last day of treatment for S201086-treated subjects but not for subjects receiving placebo. This decrease was similar for the 3 tested doses in Japanese subjects and was significant as compared to the placebo-treated subjects (see Table).
Conclusion: S201086 was shown to be safe and well-tolerated in Japanese and Caucasian healthy male subjects with a suitable PK profile and a significant decrease of the ARGS neoepitope, indicating target engagement. A global Phase 2 study to evaluate the efficacy of S201086 in patients with knee OA is ongoing (NCT03595618).
To cite this abstract in AMA style:
Lalande A, Kuzniatsova-Mouchette N, Chassereau F, Geronimi J, Larsson S, Struglics A, Lohmander S, Pueyo M. Safety, Tolerability, Pharmacokinetics and Pharmacodynamics in Healthy Male Japanese Subjects of the ADAMTS-5 Inhibitor S201086/GLPG1972, a Potential New Treatment in OA [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/safety-tolerability-pharmacokinetics-and-pharmacodynamics-in-healthy-male-japanese-subjects-of-the-adamts-5-inhibitor-s201086-glpg1972-a-potential-new-treatment-in-oa/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-tolerability-pharmacokinetics-and-pharmacodynamics-in-healthy-male-japanese-subjects-of-the-adamts-5-inhibitor-s201086-glpg1972-a-potential-new-treatment-in-oa/