Session Information
Date: Monday, November 18, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Pegloticase, infused every 2 weeks over ≥2 hrs, lowers serum urate (SU) in patients with refractory gout. Decreasing infusion time is expected to improve patient treatment experience. Methotrexate (MTX) is recommended as co-therapy to pegloticase1 to decrease pegloticase immunogenicity, subsequently increasing SU-lowering response rate and decreasing infusion reaction (IR) risk.2 Here, we report primary findings of the AGILE trial (NCT04511702) that examined pegloticase+MTX safety/efficacy with decreased pegloticase infusion time.
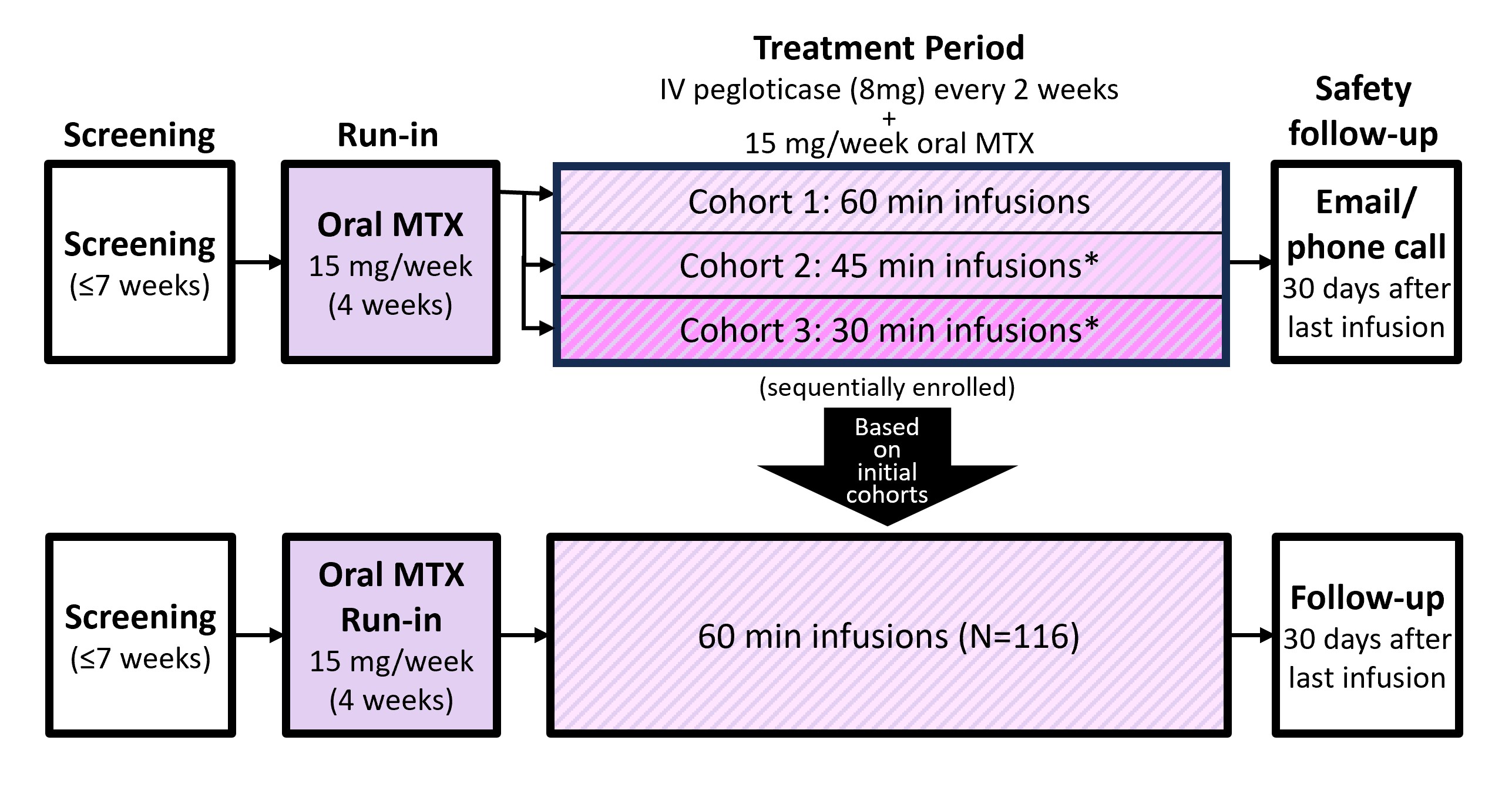
Methods: This Phase 4, multicenter, open label study included patients with uncontrolled gout (SU ≥6 mg/dL, oral urate-lowering therapy intolerance/inefficacy, and ≥1 of: ≥1 tophus, ≥2 gout flares/yr, or gouty arthritis). Key exclusion criteria were MTX contraindication, serious bacterial infection, G6PD deficiency, eGFR < 40 ml/min/1.73 m2, and elevated liver function tests. Eligible patients underwent a 4-week oral MTX run-in (15 mg/wk with 1 mg/d folic acid) followed by 24 weeks of pegloticase+MTX treatment (Day 1, first infusion). Initial cohorts were sequentially enrolled to receive 60-, 45-, or 30-min infusions to determine optimal duration for larger enrollment (Figure). The primary endpoint was the pegloticase+MTX IR rate (including anaphylaxis [AR]) of the most desirable infusion duration (110 patients planned). Key secondary endpoints were SU-lowering response rate during Month 6 (SU < 6 mg/dL for ≥80% of Weeks 20-24) and rate of pegloticase discontinuation due to IR, AR, or SU-response loss (2 consecutive SU >6 mg/dL after Week 2). Analyses included patients who received ≥1 pegloticase infusion (modified intent-to-treat [mITT]).
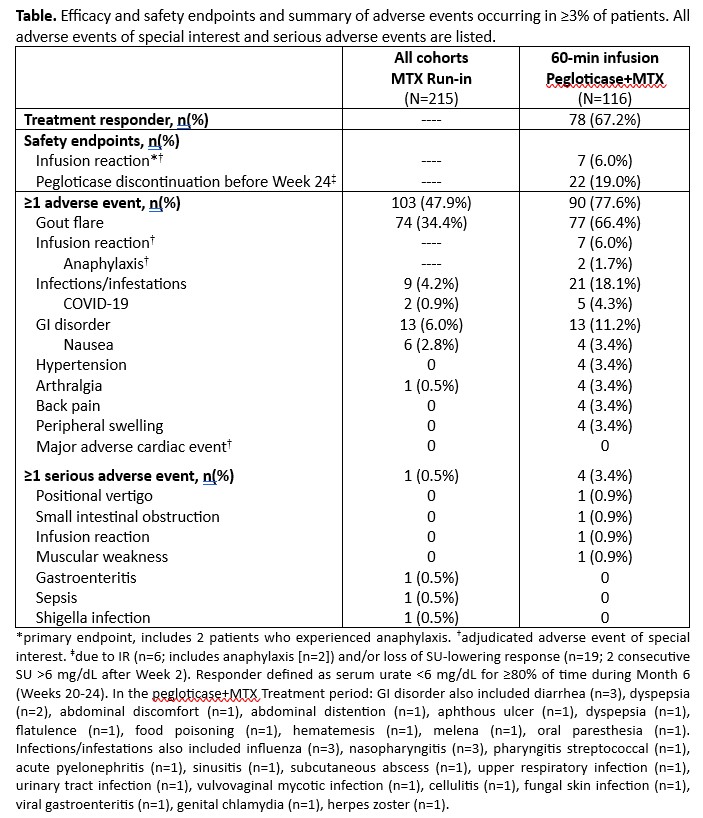
Results: Initial cohort enrollment proceeded from 60- to 45- to 30-min infusions. IR rate was highest in the 30-min cohort (12.9% [8/62]), lowest in the 45- and 60-min cohorts (both 0% [N=13, 10]). Out of caution, 60-min was chosen for further investigation. 116 patients (included initial 10 patients) made up the mITT, receiving 60-min infusions (90% men, mean [±SD] age: 55.2±11.3 yrs, BMI: 33.6±6.3 kg/m2, eGFR: 76.5±19.4 ml/min/1.73m2; 12.0±10.4 yr gout history, 66% had tophi, 7.0±7.1 flares in prior year, SU: 8.7±1.7 mg/dL). IR rate was 6.0% (95%CI: 2.5-12.0%; 7/116) in the 60-min group (including AR in 2 patients [1.7%]). The primary endpoint was met as the upper 95% CI bound for IR rate was < 13% (a pre-specified threshold) and similar to the 4.2% (including AR in 1.0%) in a prior pegloticase+MTX registrational trial with 120-min infusions (MIRROR RCT).2 SU-response rate during Month 6 (67.2% [95%CI: 57.9-75.7%] vs. 74.0%1) and overall safety profile were also similar.
Conclusion: Pegloticase treatment over 6-12 months markedly improves uncontrolled gout signs/symptoms2,3 and patient quality of life.4 However, biweekly 2-hr infusions can be challenging for patients. Safety/efficacy data from the AGILE trial, support shortening infusion time to 60-min to minimize patient treatment burden.
References
1. Pegloticase [package insert], Horizon Therapeutics USA, 2022
2. Botson JK et al. Arthritis Rheumatol 2023;75:293-304
3. Botson JK et al. ACR Open Rheumatol 2023;5:407-18
4. Botson J et al. Ann Rheum Dis. 2023;82(suppl 1):518
To cite this abstract in AMA style:
Troum O, Botson J, Fang F, Obermeyer K, Verma S, LaMoreaux B. Safety, Tolerability and Efficacy of Pegloticase Administered with a Shorter Infusion Duration in Subjects with Uncontrolled Gout Receiving Methotrexate: Primary Findings of the AGILE Open-label Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/safety-tolerability-and-efficacy-of-pegloticase-administered-with-a-shorter-infusion-duration-in-subjects-with-uncontrolled-gout-receiving-methotrexate-primary-findings-of-the-agile-open-label-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-tolerability-and-efficacy-of-pegloticase-administered-with-a-shorter-infusion-duration-in-subjects-with-uncontrolled-gout-receiving-methotrexate-primary-findings-of-the-agile-open-label-trial/