Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: There is limited data on pediatric golimumab dose escalation, with some data available only in the adult literature. The subcutaneous formulation is not approved for use in pediatric juvenile arthritis. The primary goal of this retrospective analysis was to evaluate for the safety of subcutaneous (SC) golimumab and intravenous (IV) golimumab at escalated doses in children and adolescents with autoimmune diseases. The secondary goal was to gain insight into the efficacy of escalated golimumab dosing in this population.
Methods: Patients were identified by retrospective electronic medical record review from a single center pediatric rheumatology clinic. Inclusion criteria encompassed patients with a rheumatologic disease who were treated with IV or SC golimumab at escalated doses or frequency, SC golimumab in patients under the age of 18 years, or combined SC and IV golimumab regimens. IV doses were considered escalated if patients under the age of 18 years received greater than 80 mg/m2/dose or if patients 18 years or older received greater than 2 mg/kg/dose. Increased dose frequency included those receiving IV golimumab at intervals less than every 8 weeks or SC golimumab at intervals less than every 4 weeks.
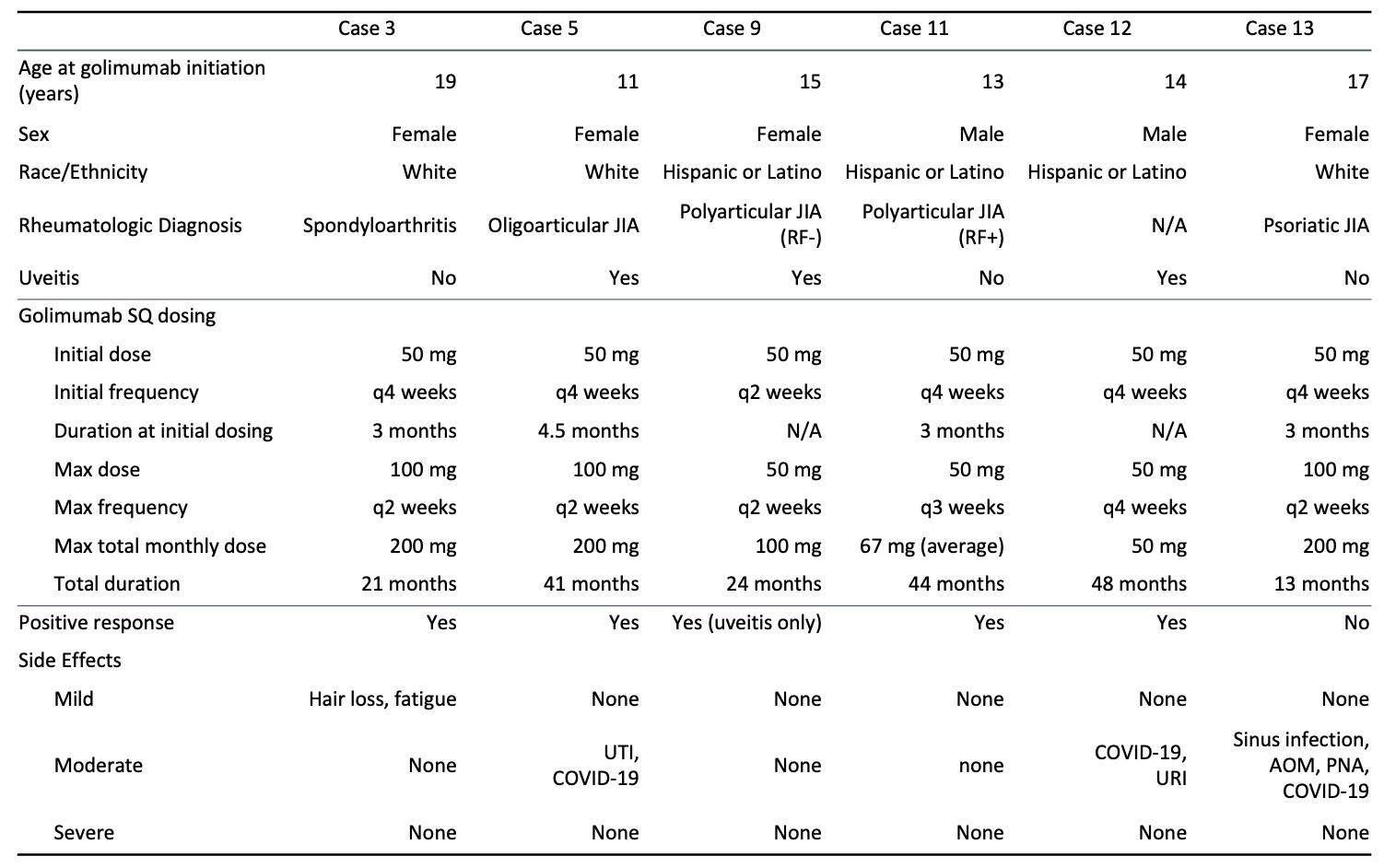
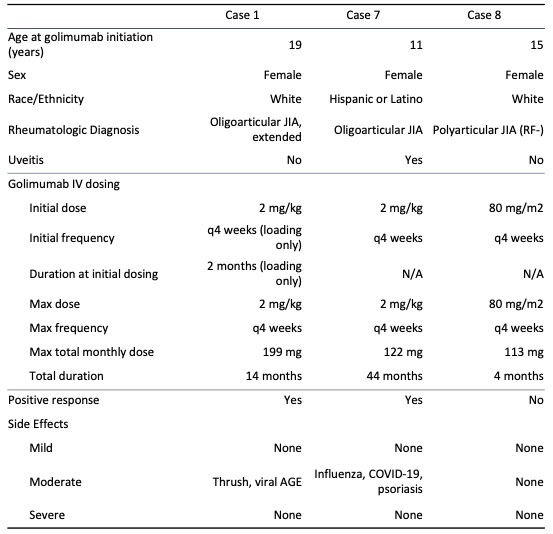
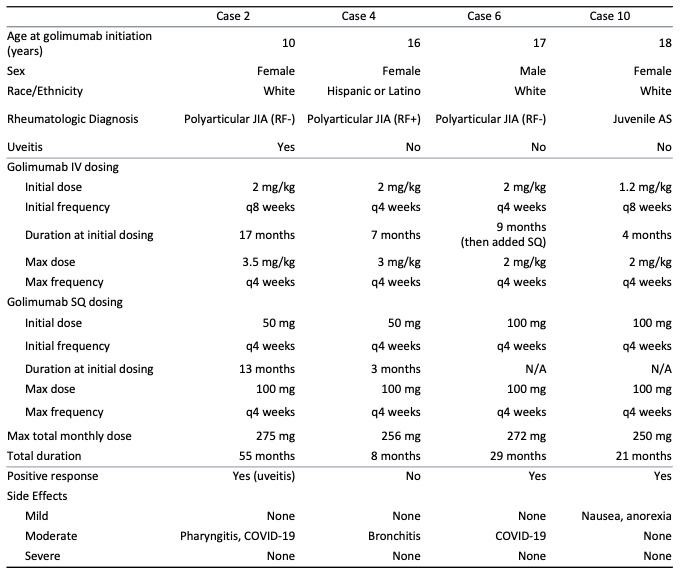
Results: Thirteen patients were identified. Ten of the patients were female and the average age at initiation of golimumab was 15 years. Average duration of treatment was 28 months. Twelve patients were treated with golimumab for management of juvenile idiopathic arthritis (JIA), 4 of which had associated uveitis, and 1 patient had uveitis without JIA. Patients had insufficient response or intolerance to an average of 2 disease-modifying antirheumatic drugs (DMARDs) and 3 biologic agents or small molecule drugs before initiating golimumab. Golimumab was the first biologic agent used in 1 patient. Positive clinical response to golimumab, based on patient report and clinician assessment, was seen in 10 patients (76.9%). Three patients discontinued high-dose golimumab due to ineffectiveness and 1 discontinued golimumab due to lack of insurance coverage. None discontinued golimumab due to side effects. Mild side effects were reported in 2 patients, including hair loss, fatigue, nausea and decreased appetite. Two patients developed psoriasis after the initiation of golimumab. Mild infections that were seen during golimumab treatment in 8 patients included upper respiratory tract infections, viral gastroenteritis, urinary tract infections, and 2 lower airway infections. There were no serious adverse events.
Conclusion: Our study suggests that dose escalation of golimumab therapy, and SC golimumab administration, are well-tolerated in pediatric rheumatologic diseases and uveitis, with a safety profile similar to that of other biologic agents. Many of our patients ( >70%) had positive clinical responses, suggesting that escalated doses may also be effective in patients with refractory disease.
To cite this abstract in AMA style:
Medrano L, Hoftman A. Safety of Golimumab Dose Escalation in Pediatric Autoimmunity: A Single Institution Retrospective Experience [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/safety-of-golimumab-dose-escalation-in-pediatric-autoimmunity-a-single-institution-retrospective-experience/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-golimumab-dose-escalation-in-pediatric-autoimmunity-a-single-institution-retrospective-experience/