Session Information
Date: Tuesday, October 28, 2025
Title: (2227–2264) Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: While disease-modifying anti-rheumatic drugs (DMARDs) are critical in managing rheumatoid arthritis (RA), their immunosuppressive effects raise concerns about cancer-related outcomes. In patients with RA who develop melanoma, evidence is limited on how post-diagnosis use of conventional synthetic (csDMARDs), biologic (bDMARDs), or targeted synthetic (tsDMARDs) DMARDs impacts mortality. This study aimed to evaluate the association between DMARD use after melanoma diagnosis and 3-year all-cause mortality in patients with RA.
Methods: This retrospective cohort study utilized data from the national Veterans Affairs (VA) Corporate Data Warehouse (CDW), spanning 2002 to 2024. Included patients had ≥2 RA International Classification of Diseases (ICD) codes within a period of 7-365 days. Patients with melanoma and their stage at diagnosis were identified through the VA Oncology Raw Domain. The main exposure was type of DMARD use, categorized as none, csDMARDs, or b/tsDMARDs (with or without csDMARDs). The primary outcome was all-cause mortality three years after melanoma diagnosis. Demographic and clinical variables–including age, sex, marital status, race, ethnicity, region, rurality, tobacco use, comorbidities, cancer stage (American Joint Committee on Cancer, AJCC)–were included. Kaplan-Meier survival analysis and multivariable Cox proportional hazards models were used to assess the association between DMARD exposure and mortality, adjusting for relevant confounders.
Results: We identified 644 patients with RA and incident melanoma. The cohort had a mean age of 71 years, with 39% aged 70-79 years. It was predominantly male (95.5%), with most residing in urban areas (57.3%). Cancer stage distribution was 46.4% Stage 0, 38.2% Stage I, 7.9% Stage II, 4.8% Stage III, and 2.6% Stage IV. Most patients entered the cohort between 2016-2021 (43.9%) (Table 1). Twelve-month post-melanoma DMARD exposure was categorized as no use (n=211, 32.8%), csDMARD use only (n=267, 41.4%), or b/tsDMARD/combination use (b/ts/Combo, n=166, 25.8%). Kaplan-Meier survival curves suggested lower three-year all-cause mortality among patients treated with b/ts/Combo compared to those receiving csDMARDs or no DMARD (Figure 1). Adjusted models showed hazard ratio (HR) of 1.12 (95% CI: 0.65–1.92) for csDMARDs relative to those on no DMARDs, and 0.65 (95% CI: 0.34–1.23) for b/ts/combination DMARDs (Figure 2).
Conclusion: In this retrospective cohort study of veterans with RA who developed melanoma, cs/b/tsDMARD use after melanoma diagnosis was not associated with increased three-year all-cause mortality. However, the study is limited by the lack of melanoma-specific mortality data and the relatively short follow-up period. These findings may help guide clinical decision-making regarding DMARD use in RA patients with a history of melanoma, particularly when balancing treatment efficacy with cancer risk.
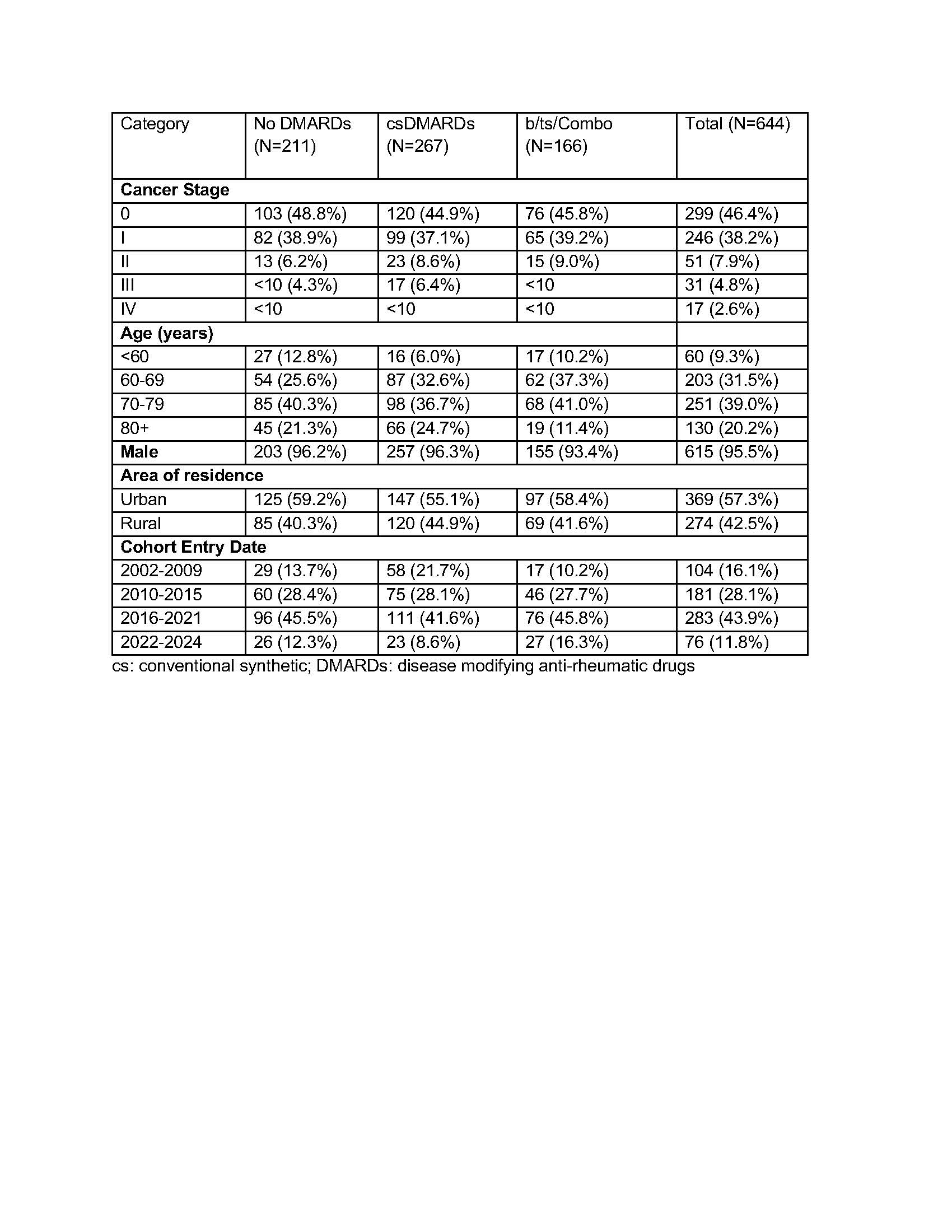 Table 1. Baseline Characteristics of Patients with Incident Melanoma by DMARD Use
Table 1. Baseline Characteristics of Patients with Incident Melanoma by DMARD Use
.jpg) Figure 1. Kaplan-Meier Survival Curves by DMARD Use Within 12 Months of Melanoma Diagnosis.
Figure 1. Kaplan-Meier Survival Curves by DMARD Use Within 12 Months of Melanoma Diagnosis.
.jpg) Figure 2. Hazard Ratio estimates (adjusted) for the association between DMARD use and three-year mortality after melanoma diagnosis in RA.
Figure 2. Hazard Ratio estimates (adjusted) for the association between DMARD use and three-year mortality after melanoma diagnosis in RA.
To cite this abstract in AMA style:
Girolami G, Kassim S, Peterson A, Baraff A, Schmidt A, Bhatia S, Miller N, Barton J, Curtis J, Li C, Singh N. Safety of DMARD therapy in veterans with rheumatoid arthritis following melanoma diagnosis: a survival analysis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/safety-of-dmard-therapy-in-veterans-with-rheumatoid-arthritis-following-melanoma-diagnosis-a-survival-analysis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-dmard-therapy-in-veterans-with-rheumatoid-arthritis-following-melanoma-diagnosis-a-survival-analysis/
