Session Information
Date: Tuesday, November 14, 2023
Title: (2039–2060) Pediatric Rheumatology – Clinical Poster III: Potpourri
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: A small proportion of children with acute COVID-19 experienced life-threatening hyperinflammation. It has been proposed that an innate immune recognition of viral RNA triggers excessive cytokine and interferon production. Adult studies have shown baricitinib usage (as part of an emergency use authorization) to be associated with stabilization of severe disease and reduced mortality. There is a paucity of pediatric data regarding COVID-19-associated JAK inhibition. We collected data on inflammation, disease progression, and safety of baricitinib in children hospitalized with COVID-19.
Methods: Retrospective medical record review was conducted of children admitted with COVID-19. Demographics, laboratory, and clinical features were collected from all children treated with baritcitinib for COVID-19 pneumonia and secondary hyperinflammation throughout all major surges of the pandemic under the auspices of an IRB approval.
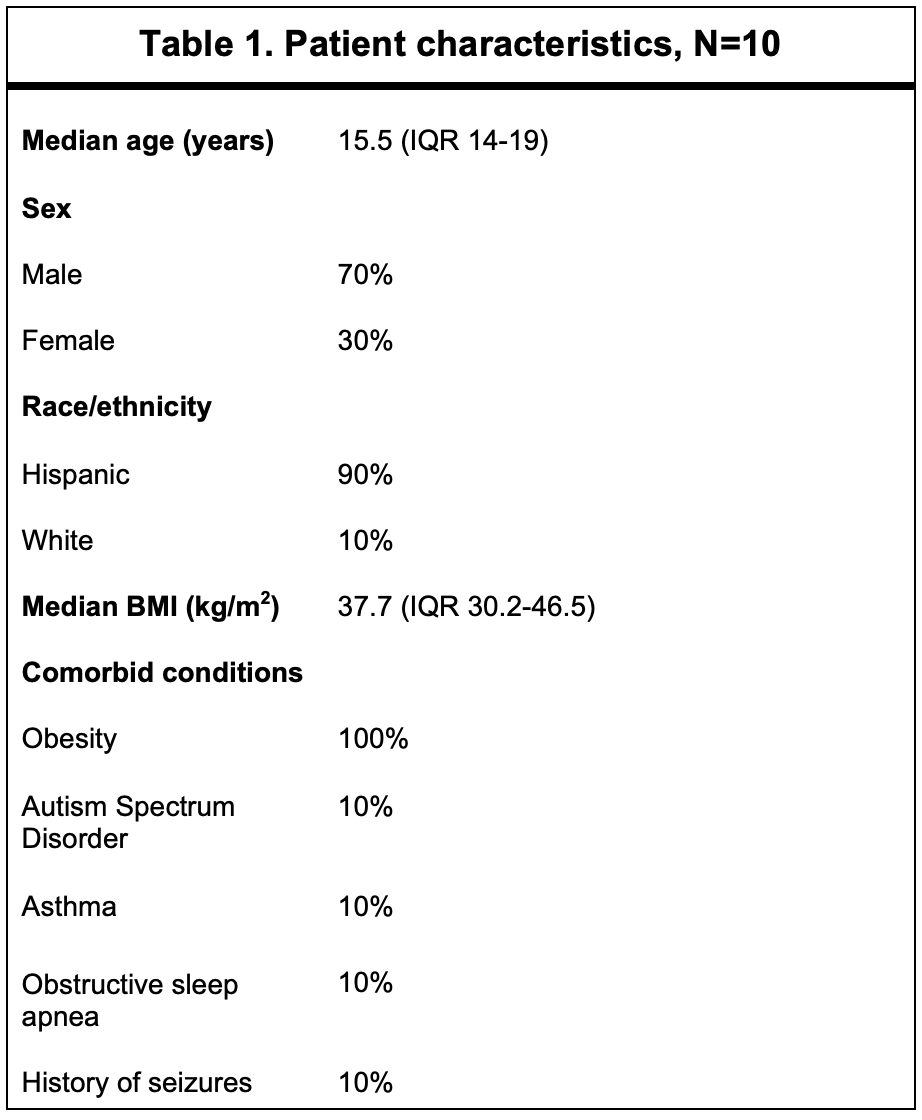
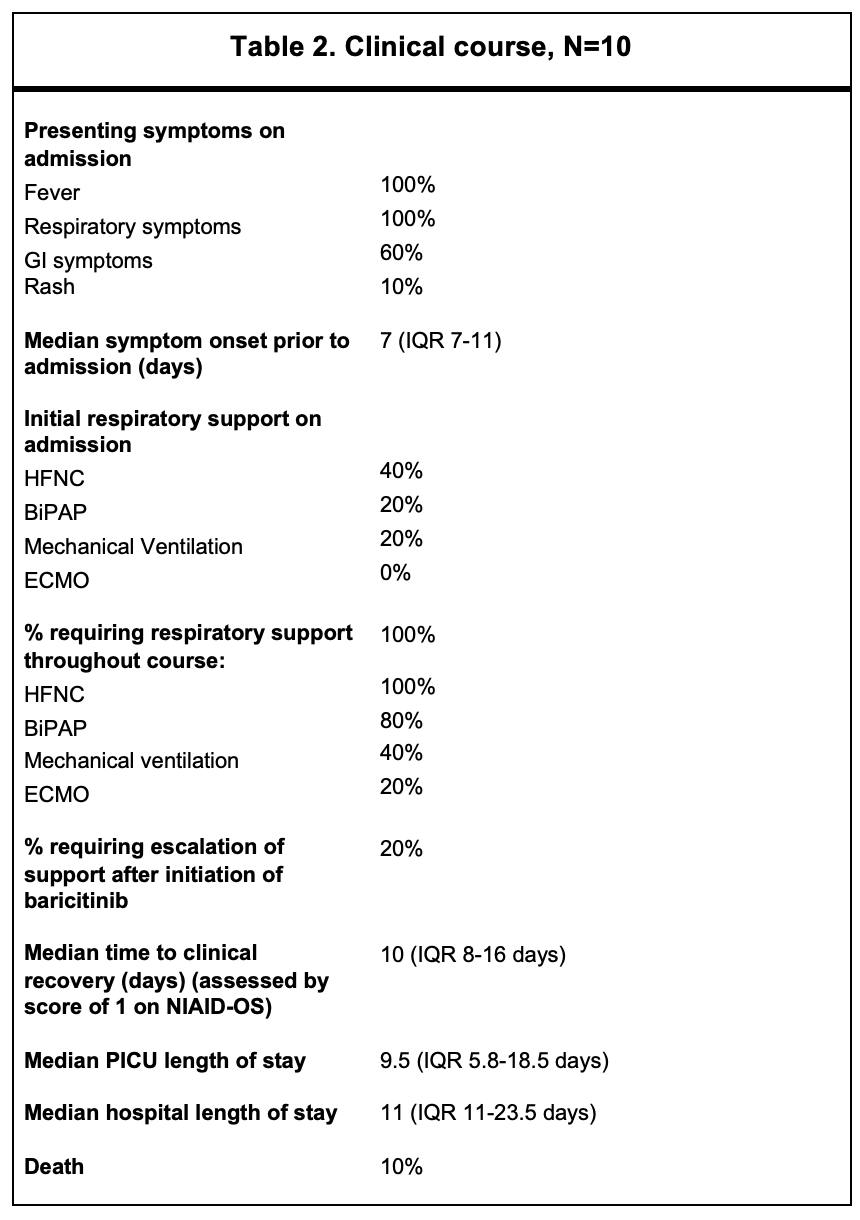
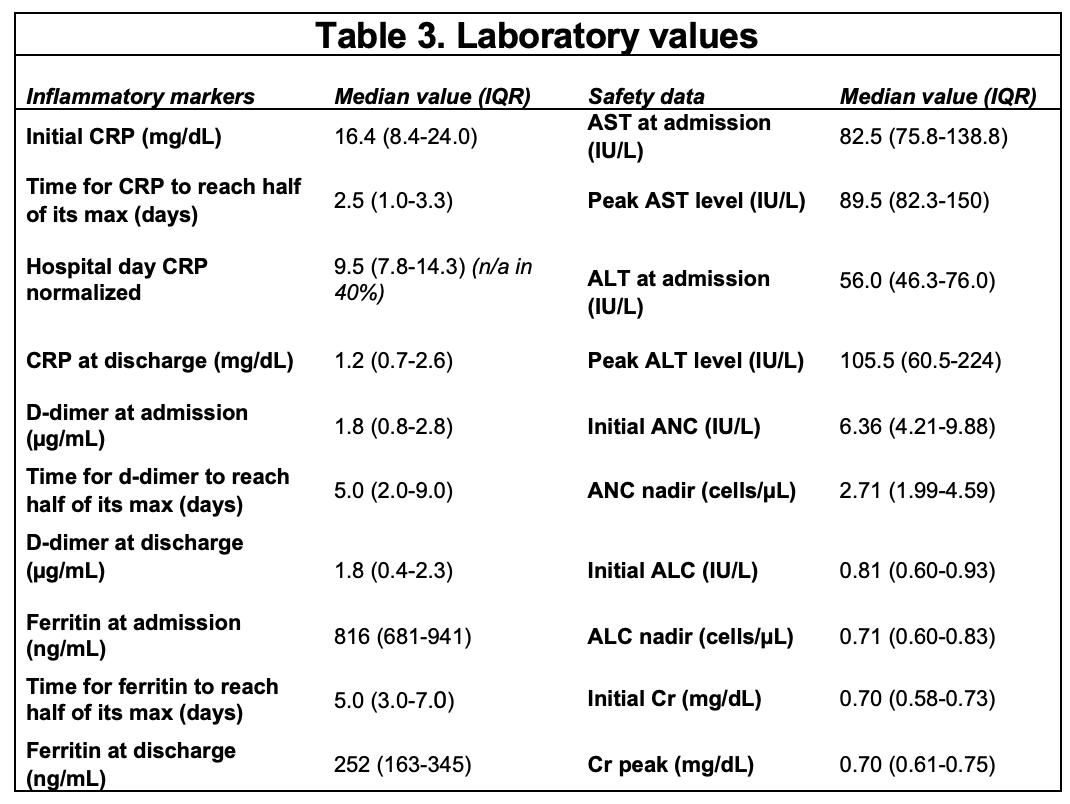
Results: The demographics and clinical features of 10 patients treated with a JAK inhibitor are shown in Table 1. Nine (90%) were Hispanic, and 7 (70%) were male. All patients were obese (BMI >95th percentile) but were otherwise healthy. All children treated with baricitinib were infected during Delta strain predominance. All had fever and respiratory distress as presenting symptoms, and 60% had GI symptoms. Median onset of symptoms was 7 days prior to admission (IQR 7-11 d). No patients met criteria for MIS-C. Baricitnib was initiated on median hospital day 2 (IQR 1.5-2 d) and administered for a median of 10 days (IQR 6-14 d). All patients were on dexamethasone and remdesivir and had improvements in their inflammatory markers during their course. Two children required escalation to ECMO after initiation of baricitinib. One of these children passed away. Of the surviving patients, median time to clinical recovery, assessed by a score of 1 on NIAID-OS, was 10 days (IQR 8-16 d). Median ICU stay was 9.5 days (IQR 5.8-18.5 d), and length of hospital stay was 11 days (IQR 11-23.5 d). Baricitinib was stopped in only one patient due to clinical concern of pulmonary embolism (unable to be imaged). Three patients developed secondary infections followingbaricitinib initiation (MSSA and culture-negative pneumonia in two). One critically ill patient developed numerous infections during the hospital stay (UTIs [E.coli and Klebsiella], MSSA pneumonia/bacteremia, Proteus mirabilis and E. faecalis wound infections). Baricitinib was safely continued with mild-moderate increased transaminases in 7 patients (Table 3). One patient developed neutropenia, and two lymphopenia. Hematologic events were transient and did not require cessation of baricitinib.
Conclusion: Children with Delta-variant associated hyperinflammation tolerated adjunctive treatment with baricitinib with a lower-than-expected adverse event and cessation rate. Additional data from other centers may be necessary to understand the role of JAK inhibition in enhancing the survival of children with severe COVID pneumonias and inflammation.
To cite this abstract in AMA style:
Rae M, Nguyen J, DeGuzman M, Foster C, Munoz F, Muscal E. Safety of Baricitinib for COVID-19 Related Hyperinflammation in Pediatric Patients: A Large Tertiary Care Center Experience [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/safety-of-baricitinib-for-covid-19-related-hyperinflammation-in-pediatric-patients-a-large-tertiary-care-center-experience/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-baricitinib-for-covid-19-related-hyperinflammation-in-pediatric-patients-a-large-tertiary-care-center-experience/