Session Information
Date: Sunday, November 13, 2022
Title: Vasculitis – ANCA-Associated Poster II: Treatment Efficacy, Clinical Outcomes, Biomarkers
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Avacopan is approved as adjunctive treatment for adults with ANCA-associated vasculitis (AAV). This study aimed to combine and report on data of the safety of avacopan from two Phase 2 and one Phase 3 studies in 439 patients with AAV.
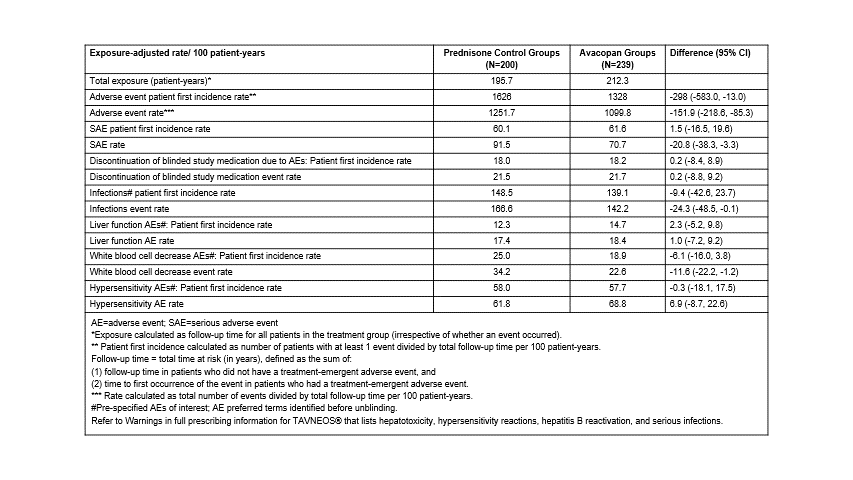
Methods: In the 3 randomized, double-blind trials, all groups received background cyclophosphamide followed by azathioprine, or rituximab; control groups received a full prednisone regimen (60 mg tapered to 0 over 20 weeks) plus placebo. The Phase 2 CLEAR trial (Jayne et al. 2017) had 3 groups: control (N=23), avacopan 30 mg twice daily (BID)+low dose prednisone (N=22), and avacopan+no prednisone (N=22). The Phase 2 CLASSIC trial (Merkel et al. 2020) had 3 groups: control (N=13), avacopan 10 mg BID (N=13), and 30 mg BID (N=16). The Phase 3 trial (ADVOCATE; Jayne et al. 2021) had a control group (N=164) and a 30 mg avacopan group with no protocol-prescribed oral glucocorticoid taper (N=166). The treatment period was 12 weeks in the Phase 2 trials and 52 weeks in the Phase 3 trial. Rates of exposure-adjusted total adverse event (AEs), serious AEs, withdrawal of study medication due to AEs, and pre-specified AEs of interest were calculated based on the integrated data from all three trials.
Results: Across the three trials, 439 patients with AAV were treated: 200 controls and 239 avacopan. Results are presented in Table 1. The rates of AE patient first incidence and AEs, serious AEs, infection events, and decrease in WBC count AEs were statistically fewer in the avacopan compared to the prednisone group.
Conclusion: In the context of avacopan’s demonstrated efficacy profile, these integrated safety results provide support for avacopan’s use in the treatment of patients with AAV.
To cite this abstract in AMA style:
Bekker P, Merkel P, Jayne D. Safety of Avacopan in ANCA-Associated Vasculitis: Combined Data from Three Clinical Trials [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/safety-of-avacopan-in-anca-associated-vasculitis-combined-data-from-three-clinical-trials/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-avacopan-in-anca-associated-vasculitis-combined-data-from-three-clinical-trials/