Session Information
Date: Sunday, November 8, 2015
Title: Osteoarthritis - Clinical Aspects Poster I: Treatments and Metabolic Risk Factors
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Knee osteoarthritis (OA) is characterized by the destruction of articular cartilage, subchrondral bone alterations and varying degrees of synovitis. Current OA treatments are limited to relieving pain as there are no drug therapies approved which treat the underlying cause of the disease. The Wnt signaling pathway is known to play a central role in the formation of joint tissues and altered Wnt signaling has been associated with cartilage loss in preclinical and clinical studies.1 SM04690 is a small molecule inhibitor of the Wnt pathway which is administered via intra-articular (IA) injection. This report provides interim safety, efficacy and biomarker data from an ongoing phase I randomized, double-blind, placebo-controlled, dose-escalation clinical trial of SM04690 in knee OA subjects.
Methods: Subjects with symptomatic, radiographic knee OA were randomized to receive a single IA injection in the target knee with either 0.03, 0.07, 0.23 mg SM04690 or placebo (volume 2mLs) in a 4:1 SM04690 (N=16):placebo (N=4) ratio. Safety, pharmacokinetics (PK), efficacy (WOMAC Total, Function, Pain subscales), and biomarker data (P1NP, β-CTXI and COMP) were collected at baseline prior to injection and during the 24-week follow-up period. Exploratory analyses of efficacy outcomes were conducted using a baseline-adjusted repeated measures analysis of covariance (ANCOVA).
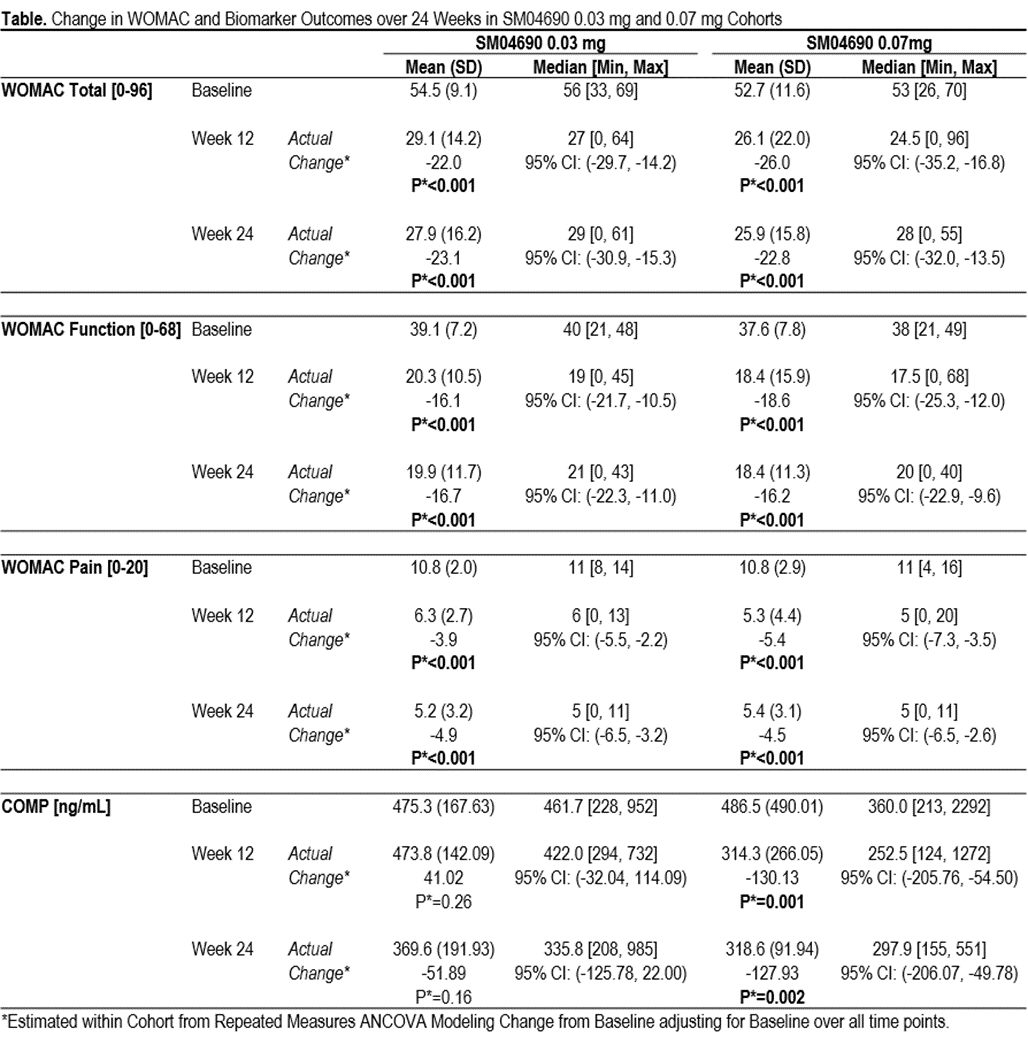
Results: 61 subjects (average age 62.6 [±5.7] years, female N=41 [67%], average BMI 30.4 [±4.7] kg/m2) were enrolled. At time of abstract submission, 41 subjects had completed the 24-week trial: Cohort 1: 0.03 mg (N=17), Cohort 2: 0.07 mg (N=16) and placebo (N=8). Cohort 3 (subjects treated with 0.23 mg [N=16] and placebo [N=4]) are ongoing and data not reported. Serum levels of SM04690 in both 0.03 and 0.07 mg groups were below limits of detection at all time points. No DLTs or SAEs were reported in the 0.03 mg cohort. 2 DLTs, paroxysmal tachycardia, (also an SAE), and increased pain were reported in 0.07 mg cohort: Both were deemed unrelated to SM04690 by safety review adjudication. 35 AEs were reported; 5 (14%) considered possibly or probably related to study drug (4 increased knee pain, 1 acne). At Week 24, improvements were seen in both 0.03 mg and 0.07 mg cohorts (respective change from baseline: WOMAC Total, -23.1 and -22.8; WOMAC Function, -16.7 and -16.2; WOMAC Pain, -4.9 and -4.5; all P<0.001) (Table). Biomarker data showed significant reduction in COMP in the 0.07 mg group at Weeks 12 (-130.13 ng/mL, P=0.001) and 24 (-127.93 ng/mL, P=0.002) (Table). There were no significant changes in COMP in the 0.03 mg group, or in β-CTX or P1NP in either 0.03 mg or 0.07 mg groups.
Conclusion: These interim data from an ongoing phase 1 trial suggest that one intra-articular injection with a novel Wnt inhibitor SM04690 into the knee in OA subjects appears safe and may be effective in reducing pain and improving function.
Reference: 1. Gelse K. Osteoarthr Cartil 2012; 20(2):162-71
To cite this abstract in AMA style:
Yazici Y, McAlindon TE, Fleischmann R, Gibofsky A, Lane NE, Kivitz AJ, Skrepnik N, Armas E, Swearingen CJ, DiFrancesco A, Tambiah JRS, Hood J, Hochberg MC. Safety, Efficacy and Biomarker Outcomes of a Novel, Intra-Articular, Injectable, Wnt Inhibitor (SM04690) in the Treatment of Osteoarthritis of the Knee: Interim, Exploratory Analysis of Results from a Randomized, Double-Blind, Placebo-Controlled Phase 1 Study [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/safety-efficacy-and-biomarker-outcomes-of-a-novel-intra-articular-injectable-wnt-inhibitor-sm04690-in-the-treatment-of-osteoarthritis-of-the-knee-interim-exploratory-analysis-of-results-from-a/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-efficacy-and-biomarker-outcomes-of-a-novel-intra-articular-injectable-wnt-inhibitor-sm04690-in-the-treatment-of-osteoarthritis-of-the-knee-interim-exploratory-analysis-of-results-from-a/