Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Dermatomyositis (DM) is a chronic systemic autoimmune disease with characteristic skin rash and muscle weakness. Intravenous immunoglobulin (IVIg) has long been used as adjuvant treatment for DM and its efficacy was recently shown in a double-blind, randomized, placebo-controlled, phase III clinical trial (ProDERM study). The aim of this sub-analysis of the ProDERM study was to present detailed safety and tolerability data of IVIg in DM subjects.
Methods: The trial enrolled adults with definite or probable DM (Bohan & Peter criteria) with active disease and muscle weakness (MMT < 142/150). In the double-blind, placebo-controlled First Period, subjects were randomized 1:1 to either high dose IVIg (2g/kg every 4 weeks) or placebo.
After week 16, all patients on placebo and patients without clinical worsening while on IVIg entered the open label Extension Period, receiving 2 g/kg IVIg infusions every 4 weeks for an additional 24 weeks period. IVIg was given in equally divided doses over 2-5 consecutive days (=1 infusion cycle) every 4 weeks.
Results: A total of 95 adult DM patients (mean age: 53 years; 75% females) were enrolled and received at least one dose of IVIg. Baseline clinical characteristics were balanced between the 2 arms. Overall 96% and 73% patients completed the first and extension period, with 3 (3%) and 9 (10%) subjects discontinuing due to adverse events (1 of 3 and 6 of 9 due to IVIg related events), respectively.
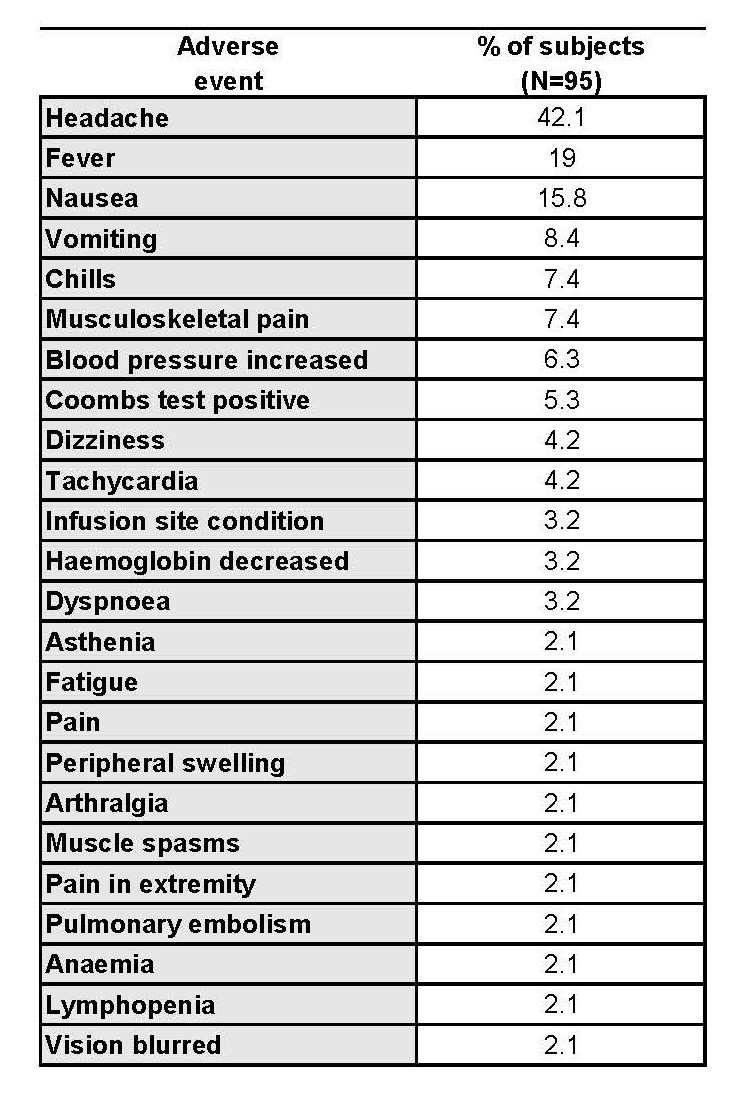
A total of 664 infusions cycles were administered and 545 adverse events (AEs) were reported. Of these AEs, 282 in 62 (65%) subjects were assessed as being related to study drug. The majority of related AEs were mild (73.4%, 207), some were moderate (23.4%, 66) or severe (3.2%, 9). Most were infusion reactions (92%, 260). Headache (42% of subjects), pyrexia (19%) and nausea (16%) were the most common AEs (Table 1). Premedication for infusions was required by only 21.3% of patients before IVIg administration.
The incidence of serious AEs was similar in the two treatment groups during the First Period: 3 subjects (5.8%) experienced 5 serious AEs after IVIg and 2 subjects (4.2%) experienced 4 serious AEs after placebo.
Overall, serious AEs were reported in 16 patients (Table 2). In 7 patients these were assessed as related to IVIg including thromboembolic events (TEE) in 5 subjects. All subjects with TEE had 1 or more other risk factors for TEE in addition to DM. During the study the exposure-adjusted incidence rate for TEE was efficiently lowered from 1.54 events per 100 patient months to 0.54 by reducing the maximum allowed infusion rate from 0.12 mL/kg/min to 0.04 mL/kg/min.
Conclusion: The safety and tolerability profile for high dose IVIg administration in patients with active DM was as expected with headache, pyrexia and nausea being most commonly reported during or after the infusions. Patients should be monitored for TEE (especially those with additional risk factors) with risk mitigation by using a low maximum infusion rate. This is the first large international, randomized, placebo-controlled phase III trial demonstrating the safety and tolerability of IVIg as a treatment for patients with DM.
Related AEs reported > 2% of subjects (First and Extension Period)
All serious adverse events (First and Extension Period) with intensity and relationship to IVIg
To cite this abstract in AMA style:
Aggarwal R, Charles-Schoeman C, Schessl J, Bata-Csorgo Z, Dimachkie M, Griger Z, Moiseev S, Oddis C, Schiopu E, Vencovsky J, Beckmann I, Clodi E, Levine T, Investigators a. Safety and Tolerability of IVIg (Octagam 10%) in Patients with Active Dermatomyositis. Results of a Randomized, Double-Blind, Placebo-Controlled Phase III Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/safety-and-tolerability-of-ivig-octagam-10-in-patients-with-active-dermatomyositis-results-of-a-randomized-double-blind-placebo-controlled-phase-iii-trial/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-tolerability-of-ivig-octagam-10-in-patients-with-active-dermatomyositis-results-of-a-randomized-double-blind-placebo-controlled-phase-iii-trial/