Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: ORAL Surveillance (NCT02092467) was mandated by the US Food and Drug Administration to assess the relative risk of tofacitinib vs TNF inhibitors (TNFi), based on observed increases in lipids and malignancies in patients (pts) with RA following tofacitinib treatment. This analysis compared the risks of major adverse cardiovascular (CV) events (MACE) and malignancies (excluding non-melanoma skin cancer) in pts with moderate to severe RA aged ≥ 50 yrs with ≥ 1 additional CV risk factor receiving tofacitinib or a TNFi.
Methods: This Phase 3b/4 prospective, randomized, open-label, non-inferiority study (co-primary endpoints: adjudicated MACE and malignancies) was in high-risk pts with RA (aged ≥ 50 yrs, ≥ 1 additional CV risk factor), receiving MTX. Pts were randomized 1:1:1 to receive tofacitinib 5 or 10 mg twice daily (BID) or a TNFi (adalimumab 40 mg every other week or etanercept 50 mg every week). Adjudicators were blinded to treatment. Outcome differences were analyzed using univariate Cox proportional hazard models. The primary comparison was whether the upper limit of the 2‑sided 95% confidence interval (CI) for the hazard ratio (HR) for tofacitinib doses combined vs TNFi was < 1.8; the secondary comparison was whether the upper limit for tofacitinib 10 vs 5 mg BID was < 2.0. Incidence rates (IRs; unique pts with events/100 pt‑yrs) were calculated for co‑primary endpoints and adverse events (AEs) of special interest. Efficacy outcomes for clinical disease activity and pt-reported outcomes were captured.
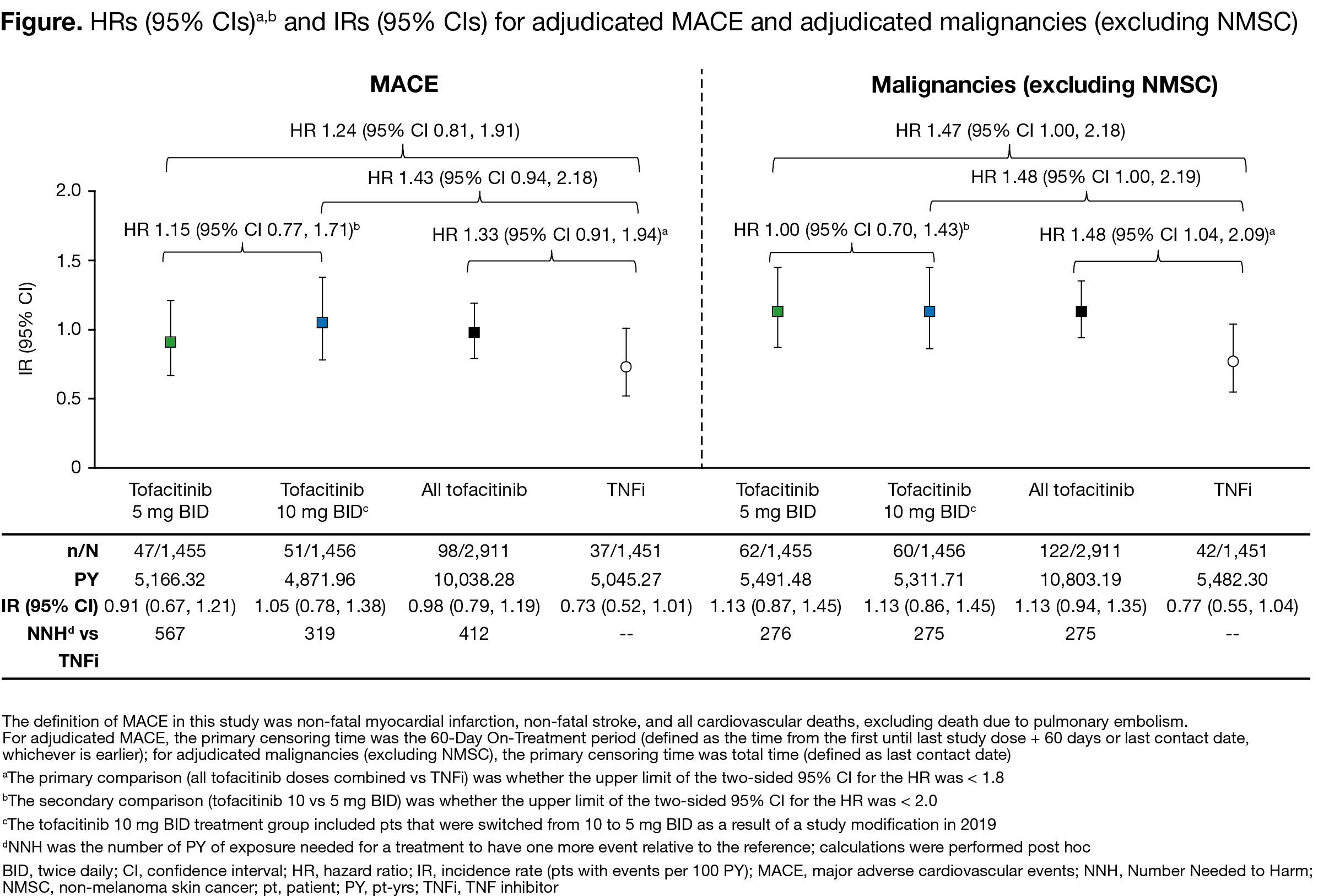
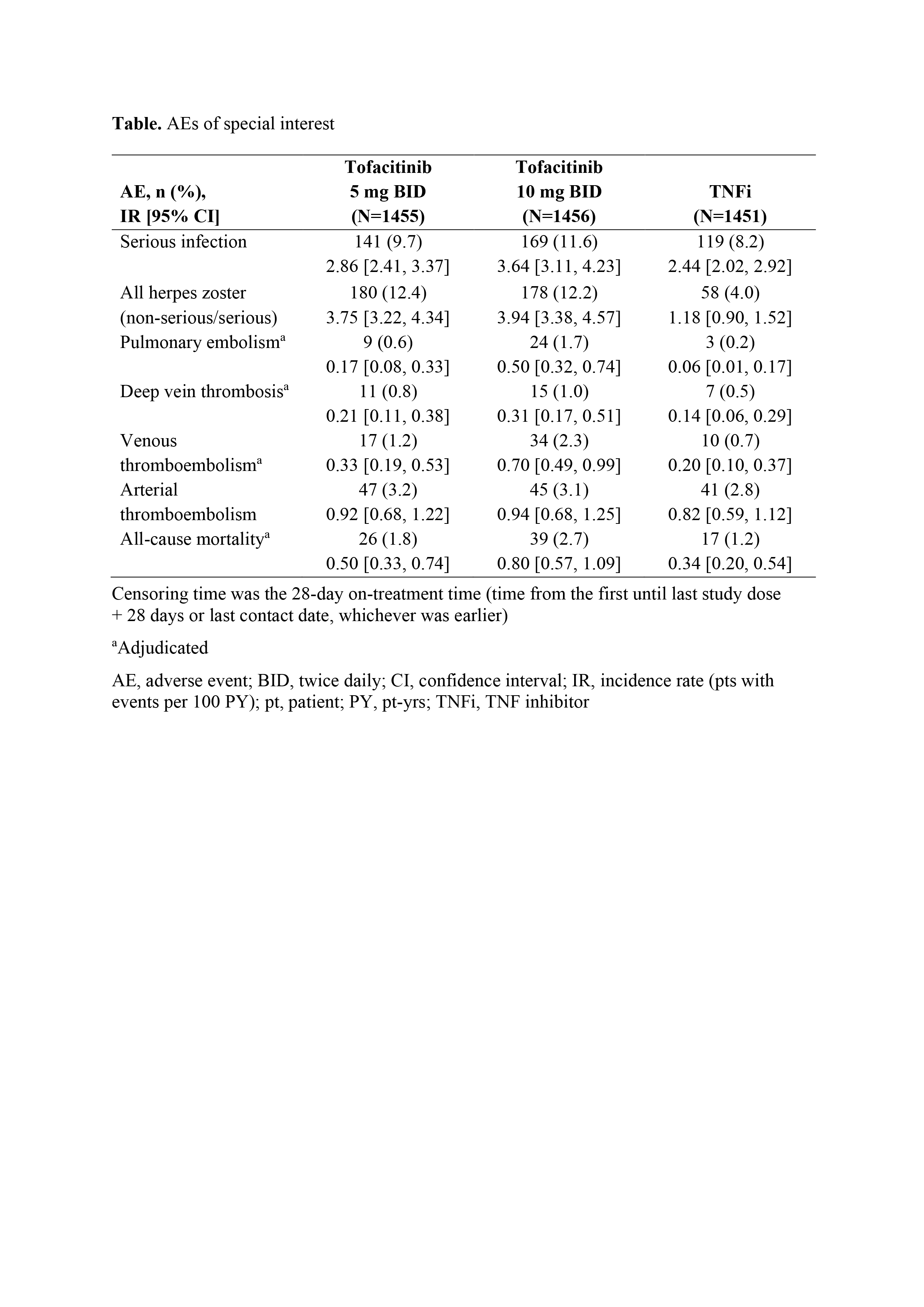
Results: Between Mar 2014–Jul 2020, 1,455, 1,456, and 1,451 pts received tofacitinib 5 mg BID, tofacitinib 10 mg BID, and TNFi, respectively. Median age was 60.0 yrs. For MACE and malignancies, primary assessments were not met for tofacitinib doses combined vs TNFi (HR [95% CI] 1.33 [0.91, 1.94] and 1.48 [1.04, 2.09], respectively; upper limits of CIs were > 1.8); for tofacitinib 10 vs 5 mg BID, the upper limits of 95% CIs were < 2.0 (Figure). IRs for MACE and malignancies were numerically higher for tofacitinib vs TNFi (Figure). In post hoc analyses, most MACE and malignancies occurred in pts who were aged ≥ 65 yrs or had ever smoked (MACE: 83/98 [84.7%] and 29/37 [78.4%]; malignancies: 102/122 [83.6%] and 33/42 [78.6%]; with tofacitinib and TNFi, respectively). IRs of AEs of special interest were numerically higher with tofacitinib (10 > 5 mg BID) vs TNFi (Table). Efficacy was similar across treatment arms.
Conclusion: For MACE and malignancies, the primary assessments were not met for tofacitinib doses combined vs TNFi in this high-risk population. Pts most at risk were aged ≥ 65 yrs or had ever smoked. IRs for MACE and malignancies were < 1.5 across all treatment groups, with overlapping 95% CIs. The Numbers Needed to Harm for tofacitinib 5 and 10 mg BID, respectively, vs TNFi were 567 and 319 for MACE and 276 and 275 for malignancies. Efficacy was similar across treatment arms.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by AG McCluskey, CMC Connect, funded by Pfizer Inc.
To cite this abstract in AMA style:
Ytterberg S, Bhatt D, Mikuls T, Koch G, Rivas J, Germino R, Menon S, Sun Y, Wang C, Shapiro A, Kanik K, Connell C, Fleischmann R. Safety and Efficacy of Tofacitinib vs TNF Inhibitors in RA Patients Aged 50 Years or Older with One or More Cardiovascular Risks: Results from a Phase 3b/4 Randomized Safety Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/safety-and-efficacy-of-tofacitinib-vs-tnf-inhibitors-in-ra-patients-aged-50-years-or-older-with-one-or-more-cardiovascular-risks-results-from-a-phase-3b-4-randomized-safety-trial/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-tofacitinib-vs-tnf-inhibitors-in-ra-patients-aged-50-years-or-older-with-one-or-more-cardiovascular-risks-results-from-a-phase-3b-4-randomized-safety-trial/