Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Autoimmune inflammatory rheumatic disease (AIIRD) patients treated with rituximab (RTX) are at risk for severe COVID19 infection, and a blunted humoral response to SARS-CoV-2 vaccines. Evusheld (AZD7442) was a combination of tixagevimab and cilgavimab, indicated for COVID19 prevention in immunosuppressed patients.
We aimed to assess the safety and efficacy of Evusheld in AIIRD patients treated with rituximab or other immunosuppressive medications.
Methods: This prospective open label longitudinal study was conducted in February-December 2022 in 3 Israeli medical centers (Tel Aviv Sourasky, Rambam, and Carmel medical centers).
Consecutive AIIRD patients over 18 years, treated with RTX or other immunosuppressive medications, and qualifying for Evusheld administration were offered to participate in the study. Patients who refused to receive Evusheld were offered to take part as a control group. The participants were followed up to 10 months after receiving the 1st 300 mg dose of Evusheld (n=78). The control group (n=39) patients were followed for the same period of time after enrollment.
Data regarding adverse events, disease activity, and COVID19, were collected 3 days and 2 weeks after enrollment, and then every month until the end of the study.
Results: The age of participants was 63.3±13.1 (mean±standard deviation (SD) years, 77.39% were females. RA was the main indication for RTX treatment (n=65, 56.52%).
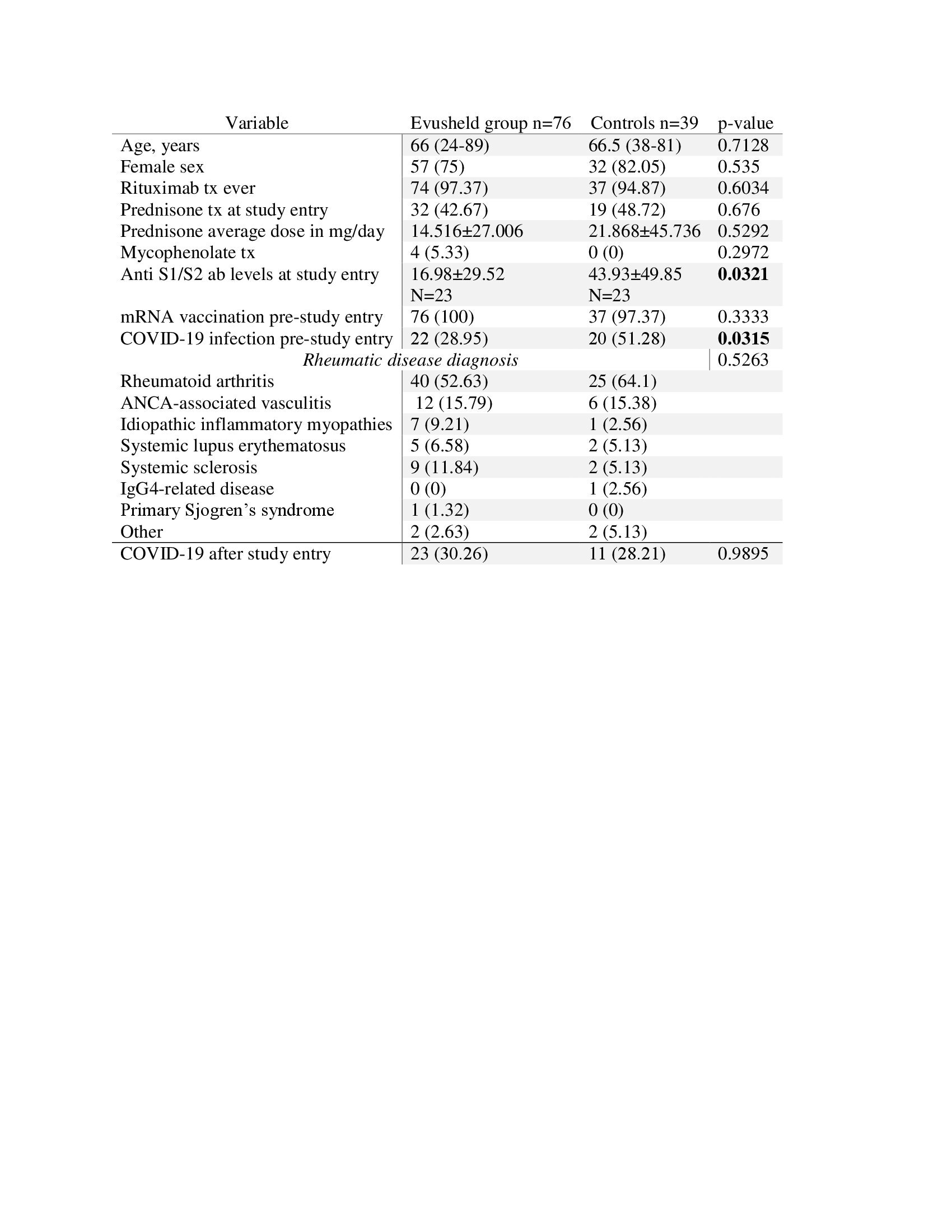
The Evusheld treated group was similar to the controls in regard to age, sex, AIIRD diagnoses, immunosuppressive treatment, and rate of mRNA vaccination (table 1). However, control group participants had a higher rate of previous COVID19 (51.28% vs. 28.95%, p=0.0315, respectively), and higher anti-S1/S2 antibody titers at study entry, compared to controls (43.93±49.85 vs. 16.98±29.52, p=0.0321, respectively).
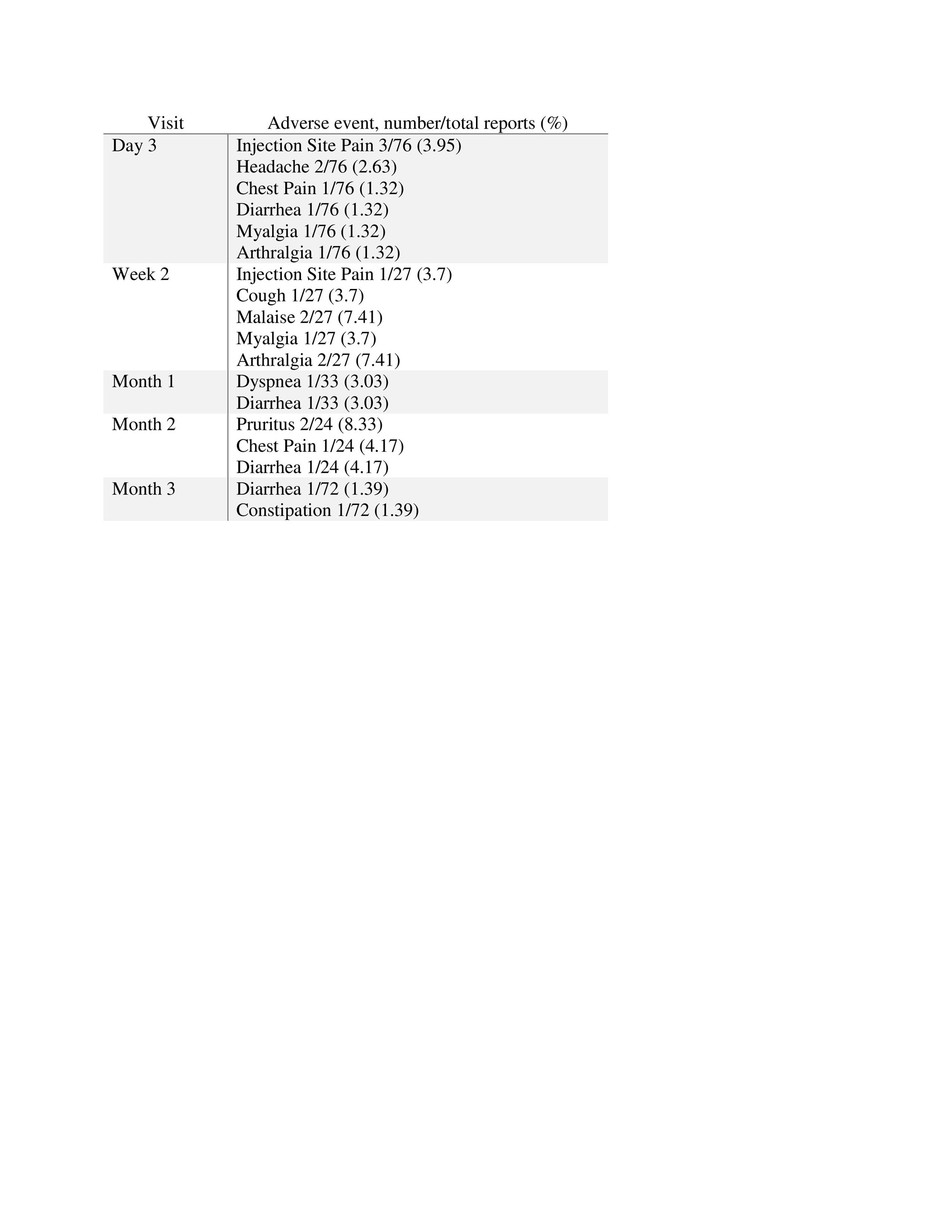
Adverse events were reported in 17.11% (n=13), most of them within 3 days following Evusheld administration, and all were mild (table 2). Injection site reactions included only pain and were reported by 3.95% of participants receiving Evusheld.
During the study, 15 patients were hospitalized, at a similar rate among the groups (10 (26.3%) Evusheld and 5 (21.7%) controls, p=0.92). One female RA patient, treated with abatacept and prednisone, died 47 days after receiving Evusheld, due to deterioration of rheumatoid lung, not considered related to the use of Evusheld.
Disease activity was generally stable and low, during the study for all indications.
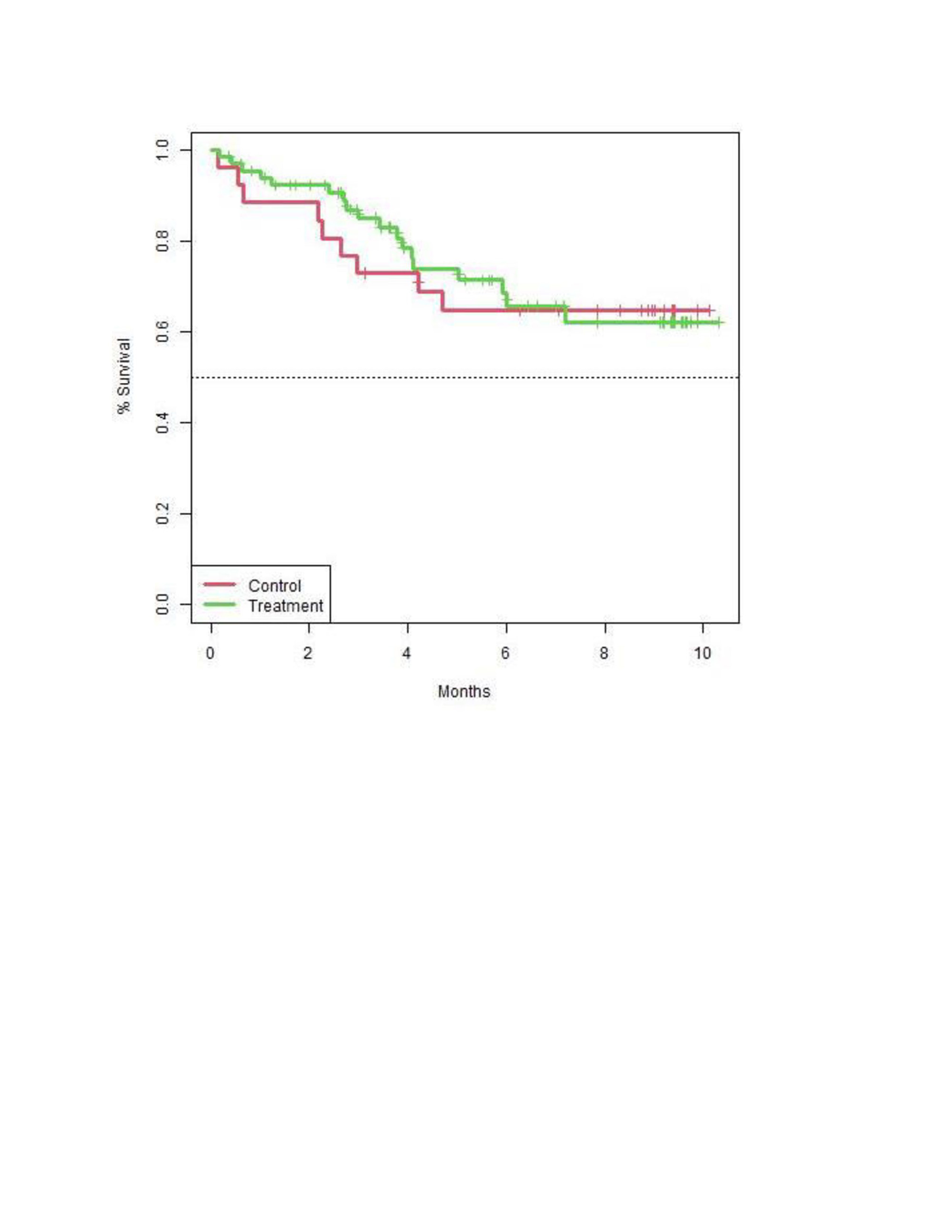
The rate of COVID19 during the study was similar between the groups (n=23, 30.3% Evusheld, n=11, 28.2% controls, p=0.99) (figure 1). Three severe infections were reported in the Evusheld group including one lethal critical COVID19 in 47 years old SSc patient who received Evusheld 6 months previously, and another 2 severe COVID19 cases. Moderate COVID19 was reported in 3 patients –2 of them were in the control group.
Conclusion: Evusheld was safe in patients with AIIRD treated with B-cell depleting or other immunosuppressives medications, that commonly fail to efficiently respond to active/mRNA vaccination. Nevertheless, Evusheld did not prove efficacious in preventing omicron and post-omicron COVID19 in general, and severe disease in particular.
Categorical variables are presented by number (percent); continuous variables are presented by mean±standard deviation, except age which is presented by median (range).
Tx, treatment; ab, antibody.
To cite this abstract in AMA style:
Eviatar T, Furer V, Kaufman I, levartovsky D, Elalouf O, Zisman D, Gazitt T, Haddad A, Elias M, Feld J, Balbir-Gurman A, Braun Moscovici Y, Pel S, Nevo s, Paran D, Elkayam O. Safety and Efficacy of Tixagevimab/Cilgavimab (Evusheld) in Autoimmune Inflammatory Rheumatic Disease Patients – a Prospective Multicenter Open-label Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/safety-and-efficacy-of-tixagevimab-cilgavimab-evusheld-in-autoimmune-inflammatory-rheumatic-disease-patients-a-prospective-multicenter-open-label-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-tixagevimab-cilgavimab-evusheld-in-autoimmune-inflammatory-rheumatic-disease-patients-a-prospective-multicenter-open-label-study/