Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Lenabasum is a rationally-designed preferential cannabinoid receptor type 2 agonist that activates resolution of innate immune responses to reduce tissue inflammation and fibrotic processes. Lenabasum had acceptable safety and tolerability and improved efficacy outcomes in the initial 16-week double-blinded, randomized, placebo-controlled Part A of Phase 2 trial JBT101-DM-001 (NCT02466243) in classic or amyopathic dermatomyositis (DM) subjects with refractory skin disease and minimal or no active muscle involvement at the time of enrollment.
Methods: To provide long-term safety and efficacy data in DM subjects in study JBT101-DM-001, subjects who completed Part A were eligible to receive oral lenabasum 20 mg BID in an open-label extension (OLE) of that study that assessed safety and efficacy at 4 weeks, then every 8 weeks.
Results: 20/22 (91%) eligible subjects received lenabasum in the OLE. Seventeen (85%) subjects were on stable baseline immunosuppressive drugs. At the time of data cut-off, 18 (90%) subjects who enrolled in the OLE had entered the second year of the OLE, and all 18 had completed Week 68.
Twenty/20 (100%) of subjects experienced at least 1 AE, with 65 total AEs occurring among the subjects during the OLE to date. The majority of AEs were mild (n = 16, 80%), and only 1 subject had an AE probably or definitely-related to lenabasum (mild fatigue). Only 1 subject had a severe AE (fatigue) and that AE was considered unrelated to lenabasum. AEs occurring in 3 (15%) subjects were dermatomyositis worsening (2 mild, 1 moderate), dizziness (all mild), fatigue (2 mild, 1 severe), nasopharyngitis (all mild). AEs occurring in 2 (10%) subjects were headache, herpes zoster, nausea, sinusitis, upper respiratory tract infection, and urinary tract infection – all were mild except 1 AE of sinusitis was moderate. No serious AEs have been reported in this study to date.
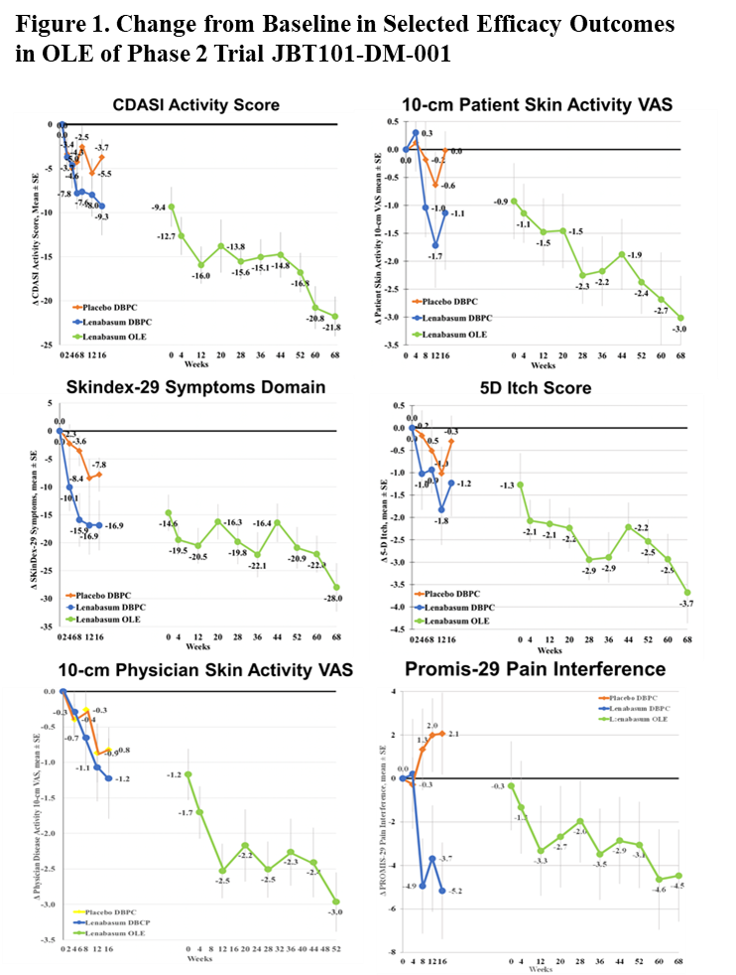
Improvement was seen in multiple physician- and patient-reported efficacy outcomes; selected outcomes are presented in Figure 1. Mean (SE) changes from study start at Week 68 in the OLE were: CDASI activity score = -21.8 (2.26), Patient Skin Activity VAS = -3.0 (0.75); Skindex-29 Symptoms Domain = -28.0 (SD); 5D Itch Score = -3.7 (SD); Physician Overall Disease VAS = -3.0 (SD); and Promis-29 Pain Interference = -4.5 (SD). Improvements were seen in other patient- and physician-reported efficacy outcomes. During the OLE, 2 subjects reduced mycophenolate, 2 were switched from methotrexate to mycophenolate, 1 started methotrexate, and 1 had a burst and taper of steroids.
Conclusion: To date, lenabasum treatment has been safe and well tolerated in the Phase 2 study JBT101-DM-001, with no serious AEs or study discontinuations related to lenabasum. The CDASI activity score and multiple other physician and patient-reported outcomes improved, although limitations of attributing efficacy to lenabasum in the setting of open-label dosing is acknowledged. These data support further testing of lenabasum for the treatment of DM, and a Phase 3 study of lenabasum in DM has started.
To cite this abstract in AMA style:
Werth V, Pearson D, Okawa J, Feng R, Concha J, Patel B, Hejazi E, Constantine S, Dgetluck N, White B. Safety and Efficacy of Lenabasum at Week 68 in an Open-Label Extension of a Phase 2 Study of Lenabasum in Refractory Skin-Predominant Dermatomyositis (DM) Subjects [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/safety-and-efficacy-of-lenabasum-at-week-68-in-an-open-label-extension-of-a-phase-2-study-of-lenabasum-in-refractory-skin-predominant-dermatomyositis-dm-subjects/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-lenabasum-at-week-68-in-an-open-label-extension-of-a-phase-2-study-of-lenabasum-in-refractory-skin-predominant-dermatomyositis-dm-subjects/