Session Information
Date: Sunday, November 10, 2019
Title: 3S084: Systemic Sclerosis & Related Disorder – Clinical I: Therapeutics & Outcomes (863–868)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Lenabasum is a synthetic, non-immunosuppressive, selective cannabinoid receptor type 2 agonist that activates resolution of innate immune responses and limits fibrosis in animal models of SSc. Lenabasum had acceptable safety and tolerability, and improved efficacy outcomes in the 16-week, double-blinded, randomized, placebo-controlled Part A of Phase 2 trial JBT101-SSc-001 (NCT02465437) in dcSSc subjects. The purpose of this study is to provide long-term open-label safety and efficacy data in study JBT101-SSc-001.
Methods: Subjects who completed Part A were eligible to receive oral lenabasum 20 mg BID in an open-label extension (OLE) that assessed safety and efficacy at 4 weeks, then every 8 weeks.
Results:
36/38 (95%) eligible subjects enrolled in the OLE. At data cut in March 2019, 29/36 (81%) patients remained in the OLE and had completed at least Week 92 in the study. Four subjects withdrew consent, 2 subjects withdrew due to AEs unrelated to lenabasum (tendonitis and scleroderma renal crisis), and 1 subject withdrew for other reasons. At Week 92, 35 (97%) subjects experienced at least 1 AE, with 249 total AEs. Seven (19%) subjects had ≥ 1 AE considered related to lenabasum in the OLE and 3 had AEs judged to be probably or definitely related to lenabasum: 1 had mild fatigue, 1 had a moderate skin ulcer and moderate lymph node pain, and 1 had mild disturbance in attention and mild lethargy and moderate feeling abnormal. Most subjects experienced AEs that were mild (n = 6, 17%) to moderate (n = 23, 64%) in maximum severity. Six (17%) had severe AEs and 1 (3%) had a life-threating AE of renal crisis associated with high-dose steroids. AEs in ≥ 10% of subjects were: upper respiratory tract infection (n = 11, 31%); skin ulcer, urinary tract infection, and arthralgia (each n = 6, 17%); and diarrhea, nasopharyngitis, and cough (each n = 4, 11%). Dizziness and fatigue occurred in 3 (8.3%) subjects each.
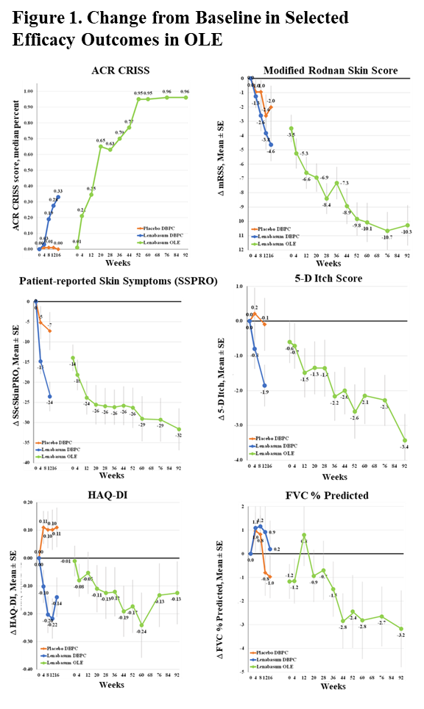
Improvement was seen in multiple physician- and patient-reported efficacy outcomes; selected outcomes are shown in Figure 1. Compared to study start, the CRISS median score (primary efficacy outcome) was 0.96 (0.43 IQR) at Week 92 and mRSS declined by mean (SD) = -10.3 (7.2) points. Health Assessment Questionnaire-Disability Index, Physician Global Assessment, Patient Global Assessment, skin symptoms, itch, and multiple PROMIS-29 domains also improved. FVC % predicted decreased 3.2% from study start.
Conclusion: Lenabasum continues to have a favorable safety and tolerability profile in the OLE of the Phase 2 trial JBT101-SSc-001 with no serious AEs or study discontinuations related to lenabasum. Efficacy outcomes show stable improvement from about 1 year in the OLE or continued improvement in some cases. Background therapy, natural history of the disease, and open-label dosing limit what can be definitely contributed to lenabasum.
To cite this abstract in AMA style:
Spiera R, Hummers L, Chung L, Frech T, Domsic R, Hsu V, Furst D, Gordon J, Myers M, Simms R, Lee E, Constantine S, Dgetluck N, Bloom B, White B. Safety and Efficacy of Lenabasum at 21 Months in an Open-Label Extension of a Phase 2 Study in Diffuse Cutaneous Systemic Sclerosis Subjects [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/safety-and-efficacy-of-lenabasum-at-21-months-in-an-open-label-extension-of-a-phase-2-study-in-diffuse-cutaneous-systemic-sclerosis-subjects/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-lenabasum-at-21-months-in-an-open-label-extension-of-a-phase-2-study-in-diffuse-cutaneous-systemic-sclerosis-subjects/