Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: RAPID-axSpA (NCT01087762) investigated the efficacy and safety of certolizumab pegol (CZP) in patients (pts) with axial spondyloarthritis (axSpA), including ankylosing spondylitis (AS) and non-radiographic (nr)-axSpA. Previously, CZP treatment has been shown to improve the signs and symptoms of axSpA over 96 weeks (wks).1
Methods: RAPID-axSpA was double-blind and placebo-controlled to Wk 24, dose-blind to Wk 48 and open-label (OL) to Wk 204. Pts fulfilled ASAS criteria and had active axSpA with positive sacroiliac joint MRI and/or raised CRP (>7.9 mg/L). Pts randomized to CZP (200 mg Q2W or 400 mg Q4W) continued their assigned dose in the OL period. Efficacy data are presented for pts originally randomized to CZP (combined doses) as observed case (OC) and with imputation: NRI for categorical measures; LOCF for continuous measures. The safety set included all pts treated with ≥1 dose of CZP.
Results: 218/325 pts were randomized to CZP from Wk 0, of whom 65% (n=142) completed to Wk 204 (AS: 67% [n=81]; nr-axSpA: 63% [n=61]). In the OL period, 9.2% of pts withdrew due to an adverse event and 1.4% due to lack of efficacy. The proportion of pts achieving ASAS20/40 and partial remission (PR) responses at Wk 24 was maintained to Wk 204 in pts remaining in the study (Figure/Table). All other clinical and patient-reported outcomes also showed maintenance of efficacy to Wk 204, with similar improvements in AS and nr-axSpA pts (Table) and in both CZP dose regimens (data not shown). Spinal mobility (BASMI-linear) and function (BASFI) also improved in both subpopulations, improvements that were maintained until Wk 204. Nr-axSpA pts had lower scores at Wk 204, but also lower levels of impairment at baseline (BL). 148 pts had BL enthesitis (MASES >0). Increasing proportions of this group who completed to Wk 204 achieved complete enthesitis clearance (MASES=0; OC): 39.6% at Wk 12, 52.5% at Wk 24, and 63.5% at Wk 204. Similarly, of 52 pts with BL heel enthesitis (tenderness at proximal insertion of ≥1 Achilles tendon; OC), 48.0% achieved clearance at Wk 12, 65.3% at Wk 24, and 74.3% at Wk 204. Pts in the safety set (N=315) had a total CZP exposure of 981 patient-years (PY), with a serious adverse event rate per 100 PY of 10.4. Event rate for serious infections was 2.3/100 PY, for malignancies 0.5/100 PY and for serious cardiovascular events 0.4/100 PY. No new safety signals were identified from Wk 96 to Wk 204, and no deaths were reported over 4 years.
Conclusion: The RAPID-axSpA trial is the first study to report on the efficacy of an anti-TNF across the broad axSpA population, including both AS and nr-axSpA pts. Long-term data from this study show that pts from both subgroups treated with CZP were able to maintain improvements in disease activity, measured both clinically and by patient-reported outcomes, with no new safety signals, over 4 years of treatment. References: 1. Sieper J. Arthritis Rheum 2015;67:668–77 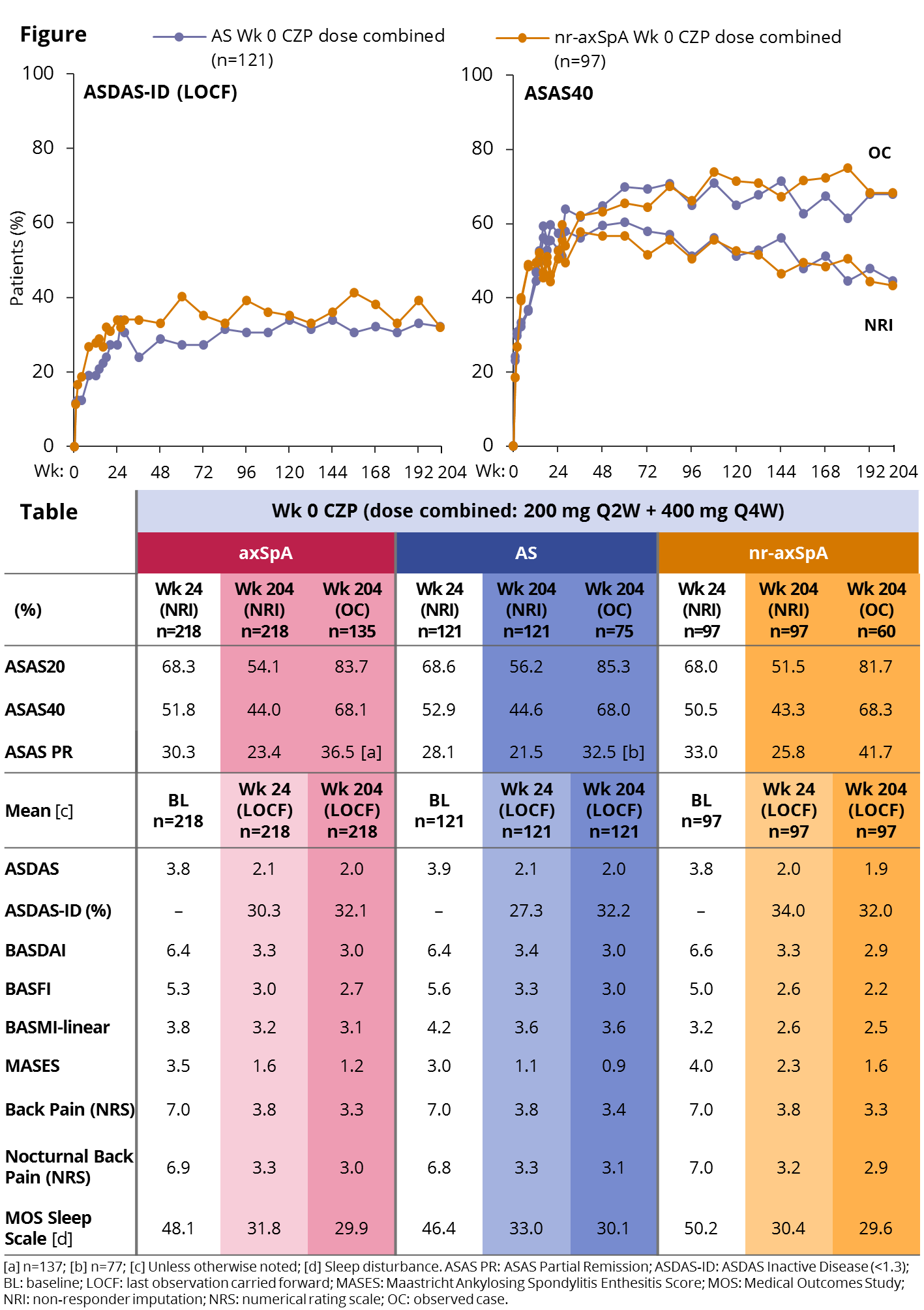
To cite this abstract in AMA style:
Deodhar AA, Dougados M, Landewé R, Sieper J, Maksymowych W, Rudwaleit M, van Den Bosch F, Braun J, Mease PJ, Kivitz A, Walsh J, Davies O, Hoepken B, Peterson L, van der Heijde D. Safety and Efficacy of Certolizumab Pegol over 204 Weeks in Patients with Axial Spondyloarthritis, Including Ankylosing Spondylitis and Non-Radiographic Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/safety-and-efficacy-of-certolizumab-pegol-over-204-weeks-in-patients-with-axial-spondyloarthritis-including-ankylosing-spondylitis-and-non-radiographic-axial-spondyloarthritis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-certolizumab-pegol-over-204-weeks-in-patients-with-axial-spondyloarthritis-including-ankylosing-spondylitis-and-non-radiographic-axial-spondyloarthritis/
