Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: A therapy transition from the originator to a biosimilar offers significant advantages for patients by lowering medication costs and improving accessibility to treatments. MSB11456 is the first tocilizumab biosimilar product approved by both FDA and EMA. The clinical development of MSB11456 relied on four studies including a Phase III, efficacy and safety study (NCT04512001, APTURA I) in moderate-to-severe Rheumatoid Arthritis (RA) patients. This Phase III study aimed to demonstrate clinical biosimilarity between MSB11456 and reference tocilizumab (EU-approved), and to evaluate whether a single treatment transition from the reference tocilizumab to MSB11456 had any impact on efficacy, or safety including immunogenicity.
Methods: RA patients were randomized to subcutaneous (SC) injections of 162 mg MSB11456 or reference tocilizumab for 24 weeks (W) (Core Period). At W24, patients receiving reference tocilizumab were re-randomized in a 1:1 ratio to continue their treatment or to switch to MSB11456 up to W52 (Transition Period). Following the transition, efficacy assessments included change from baseline in Disease Activity Score-28 Joint Count (DAS28)-erythrocyte sedimentation rate (ESR), DAS28 C‑reactive protein (CRP), ACR20/50/70 (20%, 50%, 70% improvement in American College of Rheumatology core set measures) Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI). Additional assessments included evaluation of immunogenicity at various time points up to W55 and safety evaluation up to W63. All results were summarized descriptively.
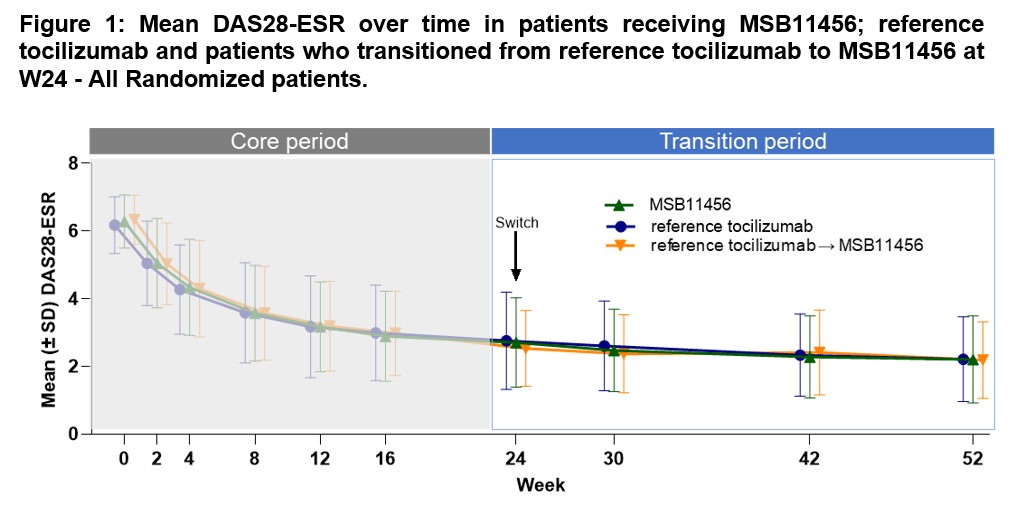
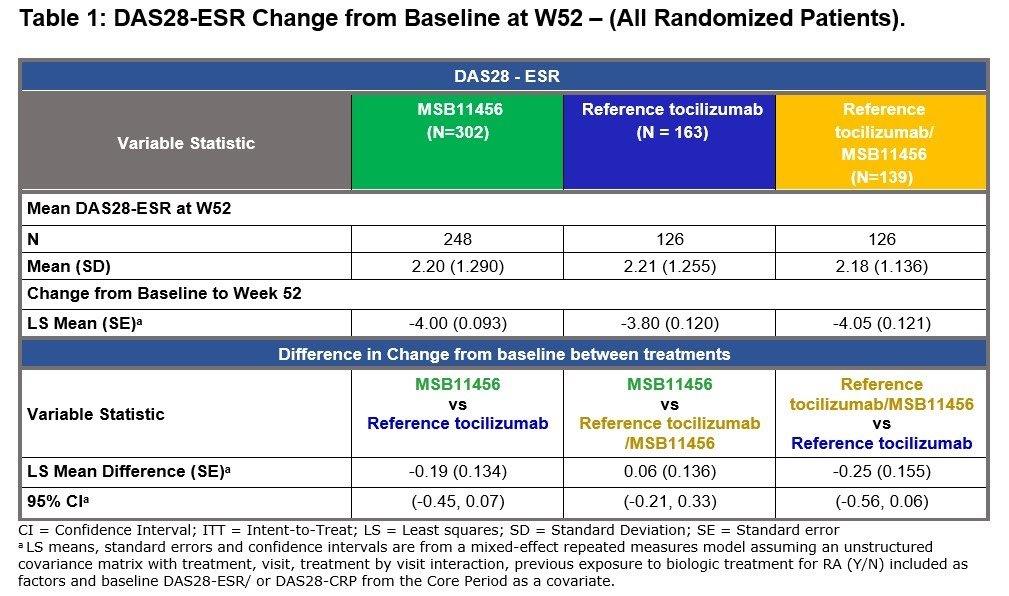
Results: Clinically relevant decreases from baseline in DAS28-ESR and in DAS28-CRP were observed as early as W2 in the two treatment groups. The magnitude of improvement observed at W24 was maintained following the transition, up to W52 (Figure 1; Table 1). Both the DAS28-ESR and DAS28-CRP mean changes from baseline at W52 were similar between the subjects who continued on reference tocilizumab and the subjects who transitioned to MSB11456 at W24. Overall, efficacy of tocilizumab was sustained up to W52 following a single transition from reference tocilizumab to MSB11456 at W24. The other efficacy endpoints analyses supported this conclusion.
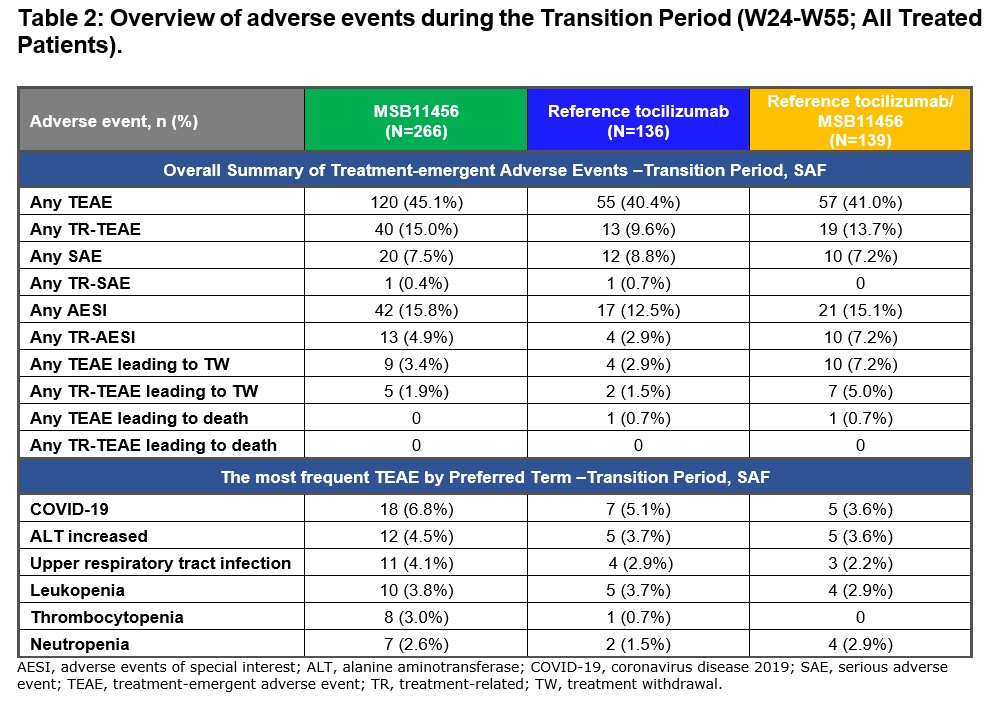
There was no notable difference in the safety profile following treatment transition from reference tocilizumab to MSB11456 compared to the patients that continued with MSB11456 or reference tocilizumab. During the Transition Period the most frequent TEAE by preferred term were COVID-19 infections, alanine aminotransferase (ALT) increase and upper respiratory tract infections (Table 2). Immunogenicity profiles were similar for the patients who transitioned treatment from reference tocilizumab to MSB11456 compared to patients who remained on reference tocilizumab or MSB11456.
Conclusion: Patients who transitioned from reference tocilizumab to MSB11456 at W24 demonstrated similar efficacy, safety, and immunogenicity during the full Transition Period up to W52 compared to the patients who continued with reference tocilizumab. Efficacy was sustained after transition with no impact on safety and immunogenicity.
To cite this abstract in AMA style:
Zubrzycka-Sienkiewicz A, Klama K, Ullmann M, Petit-Frere C, Illes A, Menang O, Monnet J, Baker P. Safe Switching from Originator Tocilizumab to MSB11456 Tocilizumab Biosimilar in Subjects with Moderate-to-Severe Rheumatoid Arthritis: Efficacy, Safety and Immunogenicity Data Following Treatment Transition in a Pivotal, Randomized, Double-blind, Phase III Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/safe-switching-from-originator-tocilizumab-to-msb11456-tocilizumab-biosimilar-in-subjects-with-moderate-to-severe-rheumatoid-arthritis-efficacy-safety-and-immunogenicity-data-following-treatment-tra/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safe-switching-from-originator-tocilizumab-to-msb11456-tocilizumab-biosimilar-in-subjects-with-moderate-to-severe-rheumatoid-arthritis-efficacy-safety-and-immunogenicity-data-following-treatment-tra/