Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Fibroblasts are key effector cells in rheumatoid arthritis (RA) underpinning joint inflammation and damage. Synovial tissue fibroblasts are heterogenous and acquire pathogenic cell states that either drive inflammation or tissue damage. However, the molecular mechanisms underpinning fibroblast heterogeneity and pathogenicity are poorly understood.
Methods: To identify key regulators of fibroblast behaviour, we performed combined scRNAseq and scATACseq analysis of synovial fibroblasts from the serum transfer induced arthritis model in mice and compared these data to additional scRNAseq data from collagen induced arthritis and antigen induces arthritis models. Ongoing validation is being performed using multiplex imaging, spatial transcriptomics (10x Visium platform), conditionally inactivating Runx1 in murine fibroblasts, lentiviral gain of function, pharmacologicalinhibition and ChIP-Seq.
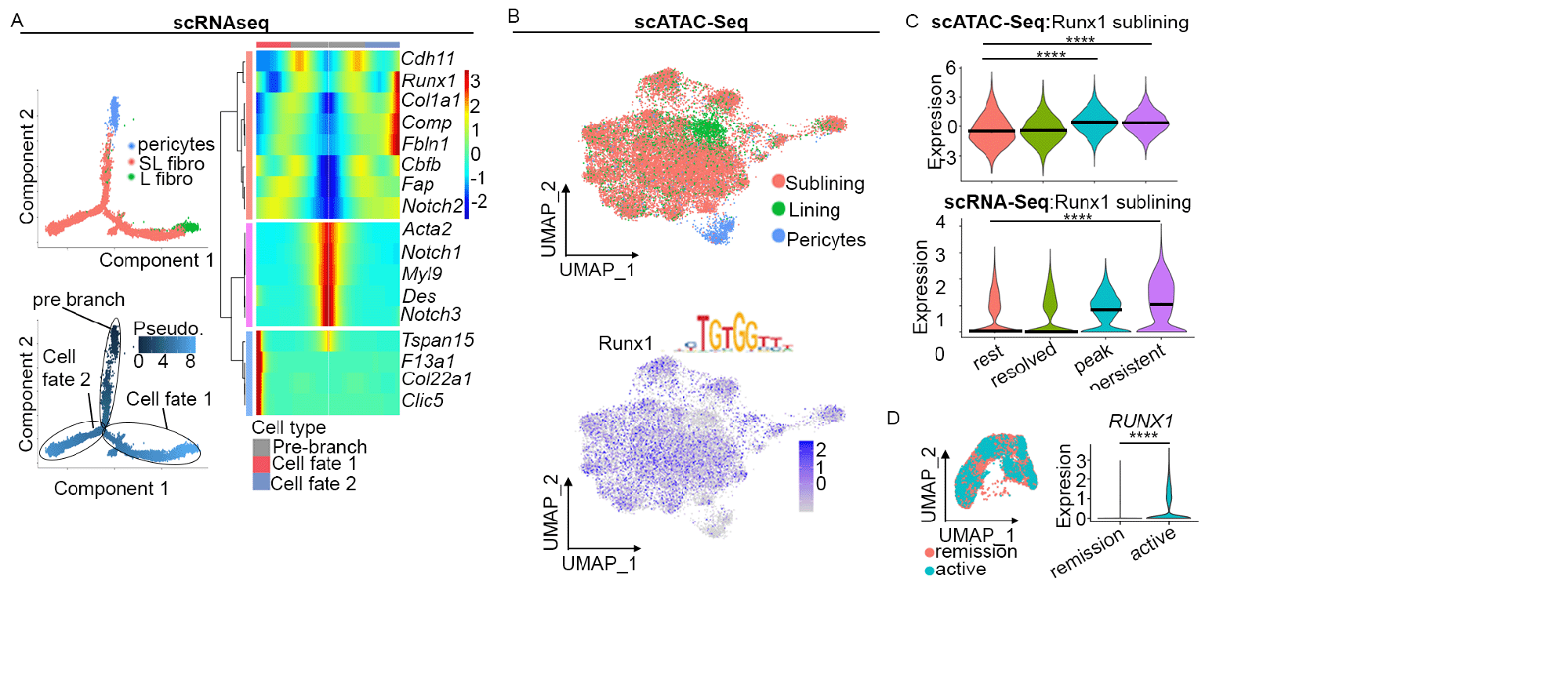
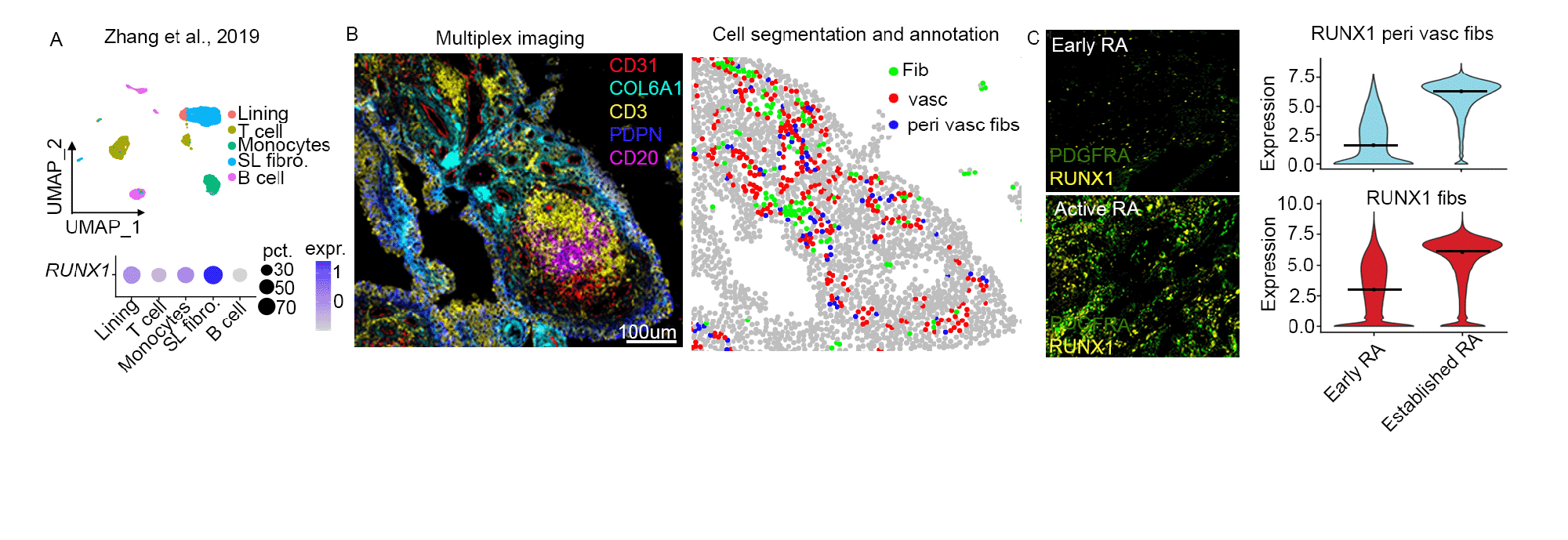
Results: We identifiedRunt-related transcription factor 1(Runx1) expression (scRNAseq) and motif activity (scATACseq) to be highly specific to sub-lining synovial fibroblasts which have been implicated in driving synovial inflammation (Fig. 1A, B). Runx1 motif activity and gene expression were also found to be upregulated during joint inflammation in mice and in patients with RA and active joint inflammation, compared to those patients in clinical remission (Fig. 1C, D). Further analysis of publicly available data from RA synovium showed robust RUNX1 expression in synovial fibroblasts (Fig. 2A). Multiplex imaging showed RUNX1 to be enriched in perivascular fibroblasts compared to interstitial fibroblasts. Furthermore, RUNX1 expression was increased in perivascular fibroblasts in active RA compared to early RA (Fig. 2B, C). We next confirmed that RUNX1 expression in synovial fibroblasts is controlled by inflammatory stimuli using confocal imaging and analysis of publicly available Hi-C data. Examining publicly available ATAC-Seq data as well as coexpression analysis using spatial transcriptomics and scRNAseq data from murine models of arthritis highlighted several direct RUNX1 target genes. We are now performing functional experiments to validate RUNX1 targets to explore how RUNX1 regulates fibroblast pathogenicity.
Conclusion: Our analysis identifies RUNX1 as a novel regulator of disease associated, synovial fibroblast behaviour in inflammatory arthritis and a potential novel therapeutic target to modulate fibroblast-driven pathology in RA.
References: Zhang F et al. Nat Immunol. 2019. PMCID: PMC6602051. Alivernini S et al. Nat Med. 2020 Aug. PMID: 32601335.
To cite this abstract in AMA style:
Mahony C, Kemble S, Chin P, Prada C, Hackland A, Reis-Nisa P, Marsh L, Keane P, Bonifer C, Buckley C, Sansom S, Coles M, Croft A. Runx1 Is a Key Transcription Factor That Drives Synovial Fibroblast Pathogenicity in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/runx1-is-a-key-transcription-factor-that-drives-synovial-fibroblast-pathogenicity-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/runx1-is-a-key-transcription-factor-that-drives-synovial-fibroblast-pathogenicity-in-rheumatoid-arthritis/