Session Information
Date: Monday, October 27, 2025
Title: Abstracts: Systemic Sclerosis & Related Disorders – Clinical II (0879–0884)
Session Type: Abstract Session
Session Time: 11:15AM-11:30AM
Background/Purpose: Systemic sclerosis (SSc) is a rare disease that often leads to severe complications and premature mortality. Recent advancements in the field have led to the emergence of more intensive therapy strategies for refractory or severe SSc patients, including haematopoietic stem cell transplantation (HSCT) and chimeric antigen receptor T-cell (CAR T-cell) therapy. Nevertheless, these therapeutic interventions are associated with considerable risks, including treatment-related mortality (TRM). In order to identify patients who may benefit from such therapies, a reliable mortality risk score is required to allow us to identify patients for whom a high mortality risk justifies the considerable TRM.
Methods: With RESIST (Risk score for Early mortality to stratify for Intensive SSc Therapy) we aimed to develop a simple risk score to predict early mortality (< 5 years) in SSc patients eligible for intensive therapies using the EUSTAR cohort. Patients deemed unsuitable for intensive therapies were defined as patients over the age of 65 years, with a history of renal crisis, LVEF < 40%, DLCO < 40%, or FVC < 45% [1]. Only patients with at-least one follow-up visit were included.The overall survival was estimated by the Kaplan-Meier method. We compared SSc patients who had succumbed during the initial five-year period with those who had survived until the 5th year. Covariates were selected according to expert opinion, univariable models, and evidence from the literature. A multivariable Cox model with adaptive LASSO variable selection was developed using 20 imputed datasets. Model performance was assessed via C-index, AUC at 3 and 5 years, and calibration. Internal validation by bootstrapping corrected for optimism using the calibration slope
Results: From 22,059 patients who were included in the EUSTAR data base, a total of 4,526 patients were included following the application of exclusion criteria. 3797/4172 met the ACR/EULAR criteria for SSc (354 missing data) Median follow-up was 53 months (95%CI: 51-55 months). The 5-year survival rate was 98% [95%CI: 97.6-98.5]. Baseline characteristics are presented in Table 1. The RESIST score included the following eight characteristics: male sex, dcSSc, age >55 years, elevated CRP, digital ulcers (current or previous), mRSS >14, LVEF < 60% and DLCO-SB < 60% (Table 2) The AUC was 0.80 and 0.79, respectively for the 3 and 5-year mortality. As demonstrated in Figure 1, the three-cut-off system (low: 0; intermediate: 1-22; high risk: >22 points) enables an effective stratification of patients into different risk categories.
Conclusion: This future mortality risk score has the potential to serve as a valuable tool to stratify patients who could benefit from intensive (cellular) therapies in SSc patients with an acceptable benefit-risk profile. The identification of high-risk patients by clinicians is of paramount importance, as it enables the optimization of treatment plans and the improvement of overall survival.
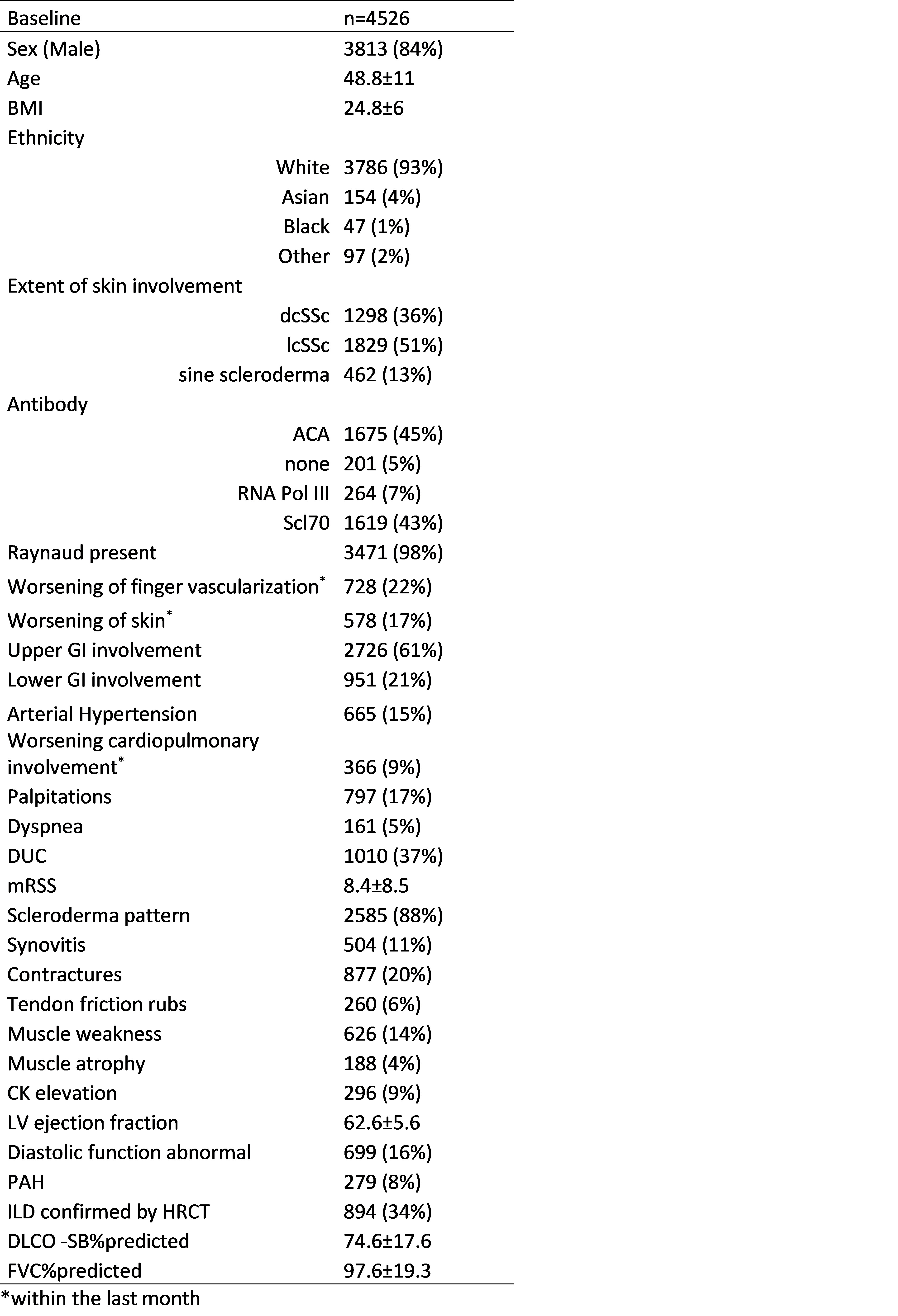 Table 1: Baseline characteristics
Table 1: Baseline characteristics
.jpg) Table 2: Multivariate analysis. The simplified score was utilised to differentiate patients into distinct categories: high (>22), intermediate (1-22), and low (0).
Table 2: Multivariate analysis. The simplified score was utilised to differentiate patients into distinct categories: high (>22), intermediate (1-22), and low (0).
.jpg) Figure 1: Overall survival according to simplified score. The 5-year survival rate was 99% (95% CI: 98–100) for low-risk patients, 96% (95% CI: 95–97) for intermediate-risk patients, and 82% (95% CI: 78–87) for high-risk patients.
Figure 1: Overall survival according to simplified score. The 5-year survival rate was 99% (95% CI: 98–100) for low-risk patients, 96% (95% CI: 95–97) for intermediate-risk patients, and 82% (95% CI: 78–87) for high-risk patients.
To cite this abstract in AMA style:
Pecher A, Marouane B, Distler O, Smith V, de Vries-Bouwstra J, Bečvář R, Moroncini G, Launay D, Allanore Y, De Santis M, Solanki K, Montecucco C, Idolazzi L, Fathi N, Kotyla P, Elhai M, Henes J. Risk Score for Early Mortality to stratify for Intensive SSc Therapy [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/risk-score-for-early-mortality-to-stratify-for-intensive-ssc-therapy/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-score-for-early-mortality-to-stratify-for-intensive-ssc-therapy/
