Session Information
Date: Monday, November 9, 2020
Title: RA – Treatments II: Potential Harms & Adverse Events (1998–2002)
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: Prior studies suggest an increased risk of venous thromboembolism (VTE) among patients with rheumatoid arthritis (RA). However, little is known about the comparative risk of VTE associated with the 1st line conventional disease-modifying antirheumatic drugs (DMARDs), methotrexate (MTX) and hydroxychloroquine (HCQ). The objective of this study was to compare the incidence rate of VTE in RA patients who were newly treated with MTX versus HCQ.
Methods: Using Medicare claims data (Parts A/B/D 2008-2017), we identified RA patients who were new users of MTX and HCQ and had no prior use of any DMARDs. Patients were required to be ≥65 years old at the cohort entry (i.e. MTX or HCQ initiation date) and have ≥365 days of continuous insurance enrollment. Patients with a diagnosis of VTE, cancer (except non-melanoma skin cancer), or lupus, or use of anticoagulants or chloroquine at baseline were excluded. The primary outcome was VTE, defined as an inpatient primary diagnosis of pulmonary embolism (PE) or deep vein thrombosis (DVT). Secondary outcomes included VTE defined as an inpatient or outpatient diagnosis of PE or DVT plus a dispensing of anticoagulants within 30 days, and separate PE and DVT based on an inpatient primary diagnosis. Using as-treated analysis, the study participants were followed from one day after the cohort entry until the earliest event of the outcome, treatment discontinuation or switch, insurance disenrollment, death, or end of data. To control for confounding, MTX and HCQ new users were 1:1 matched on the propensity score (PS) that was estimated based on patient baseline demographics, comorbidities, medication use, medical procedures, and healthcare utilization. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazards models.
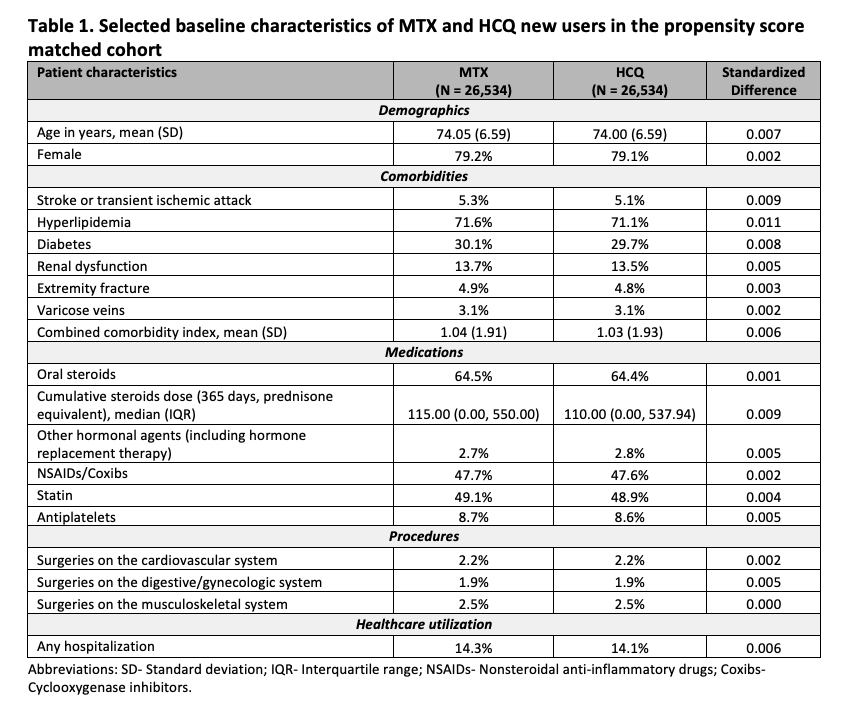
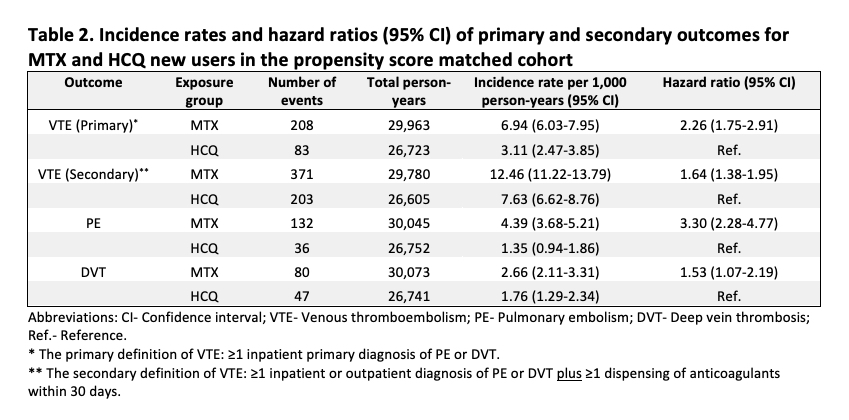
Results: We identified a total of 26,534 PS-matched pairs of MTX and HCQ initiators. Their mean (standard deviation) age was 74 (7) years and 79% were female. We observed a slightly higher proportion of steroids use in the MTX group before PS matching, but the patient baseline characteristics in the PS matched cohort were well balanced between the two treatment groups (Table 1). In the primary analysis, 208 MTX and 83 HCQ new users developed VTE during a median follow up of 190 days (interquartile range 93-452 days). The incidence rate (per 1,000 person-years) was 6.94 (95% CI 6.03-7.95) in the MTX group and 3.11 (95% CI 2.47-3.85) in the HCQ group (Table 2). The HR for incident VTE comparing MTX with HCQ initiators was 2.26 (95% CI 1.75-2.91) using the primary definition and 1.64 (95% CI 1.38-1.95) using the more sensitive but less specific secondary definition. The results were consistent in the analyses of PE and DVT, separately (Table 2).
Conclusion: In this large cohort of older RA patients, we observed an approximately 2-fold increased risk of VTE among patients who were newly treated with MTX versus HCQ. The comparative thrombotic safety profile of MTX and HCQ should be considered when physicians treat newly diagnosed RA patients with known VTE risk factors.
To cite this abstract in AMA style:
He M, Pawar A, Desai R, Glynn R, Lee H, Weinblatt M, Solomon D, Kim S. Risk of Venous Thromboembolism in Rheumatoid Arthritis Patients Treated with Methotrexate versus Hydroxychloroquine [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/risk-of-venous-thromboembolism-in-rheumatoid-arthritis-patients-treated-with-methotrexate-versus-hydroxychloroquine/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-venous-thromboembolism-in-rheumatoid-arthritis-patients-treated-with-methotrexate-versus-hydroxychloroquine/