Session Information
Session Type: Abstract Session
Session Time: 1:00PM-1:15PM
Background/Purpose: In older adults with rheumatoid arthritis (RA), anti-TNF therapies are typically used as first-line biologic treatment. However, many patients require a switch to non-TNF biologics due to inadequate response or adverse effects. While the infection risk associated with initial anti-TNF therapy is well-established, less is known about the safety of non-TNF therapies initiated after anti-TNF exposure in this population. This uncertainty is particularly relevant for late-onset RA (LORA), where age-related immune changes and distinct disease characteristics may increase infection risk. This study examines the risk of serious infection (SI) associated with initiating non-TNF biologics after anti-TNF therapy in older adults with RA, comparing outcomes between those with LORA and younger-onset RA (YORA).
Methods: Using data from FORWARD—The National Databank for Rheumatic Diseases (2001–2019), we identified RA patients aged ≥60 years who initiated a non–TNF b/tsDMARD (anakinra, abatacept, JAK inhibitors, tocilizumab, rituximab) following anti-TNF therapy. Patients were categorized as LORA or YORA based on RA diagnosis age (≥60 vs < 60) and propensity score–matched on age at treatment, sex, race/ethnicity, and comorbidities. Those with other rheumatic diseases, cancer, or HIV before the first SI event were excluded. The index date was the first report of subsequent treatment after anti-TNF use. SI was defined as infection requiring IV antibiotics, hospitalization, or resulting in death within 12 months of the index date and was attributed to subsequent therapy if it occurred during treatment or within three months of discontinuation (extended to 12 months for rituximab). SI risk differences were estimated using kernel-weighted, propensity score–adjusted survival models, stratified by RA onset group.
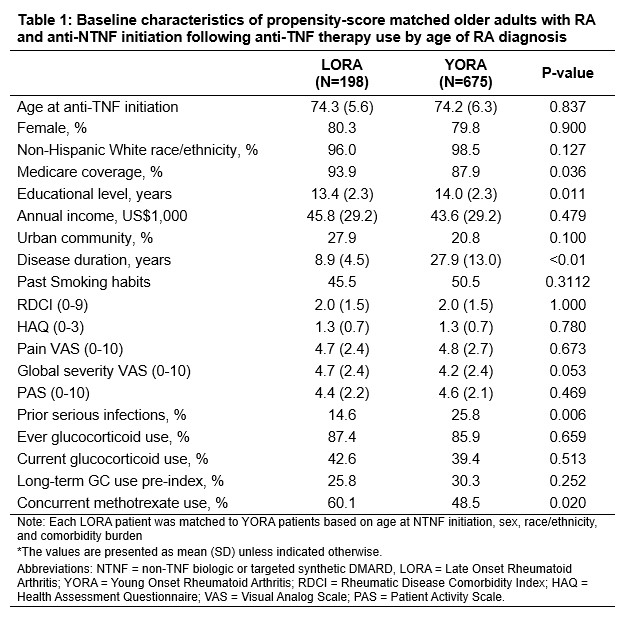
Results: 198 LORA patients were matched to 675 weighted YORA patients. Baseline characteristics after kernel propensity-score matching are summarized in Table 1. Abatacept was the most commonly used non-TNF b/tsDMARD in both groups (49.1% in YORA, 47.7% in LORA), followed by rituximab (22.6% in YORA, 22.2% in LORA). A greater proportion of LORA patients initiated subsequent therapy after only one prior anti-TNF agent compared to YORA (81.5% vs. 68.2%). The crude incidence of SI was 1.4 per 1,000 patient-years in YORA and 2.0 per 1,000 patient-years in LORA. In Cox regression models, the risk of SI was higher with older age groups at non-TNF therapy initiation, prior infection, higher HAQ disability score, and recent long-term GC use (Table 2). The risk of SI did not differ significantly between abatacept and other non-TNF therapies.
Conclusion: Among older adults with RA who initiated a non-TNF b/tsDMARD after anti-TNF therapy, the risk of SI did not differ by RA onset age or by specific treatment regimen (abatacept vs. other non-TNF therapies). However, prior SI, recent long-term GC use, and higher HAQ disability scores were independently associated with increased SI risk. These findings highlight the importance of individualized infection risk assessment when selecting second-line therapies in older adults.
To cite this abstract in AMA style:
Lee J, Pedro S, Michaud K. Risk of Serious Infection associated with non-TNF biologic initiation after Anti-TNF Use in Older Adults with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/risk-of-serious-infection-associated-with-non-tnf-biologic-initiation-after-anti-tnf-use-in-older-adults-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-serious-infection-associated-with-non-tnf-biologic-initiation-after-anti-tnf-use-in-older-adults-with-rheumatoid-arthritis/

.jpg)