Session Information
Date: Sunday, November 17, 2024
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Rheumatoid arthritis (RA) is associated with various comorbidities and complications among which cancer has been highlighted in literature. This cancer risk has been attributed to the disease process of RA as well as to DMARDs such as methotrexate, and biologics such as TNF-α inhibitors. Our objective was to perform a systematic review and meta-analysis of randomized controlled trials and observational studies to assess possible risk of cancer in patients with RA on TNF-α inhibitors, compared to patients not exposed to TNF-α inhibitors.
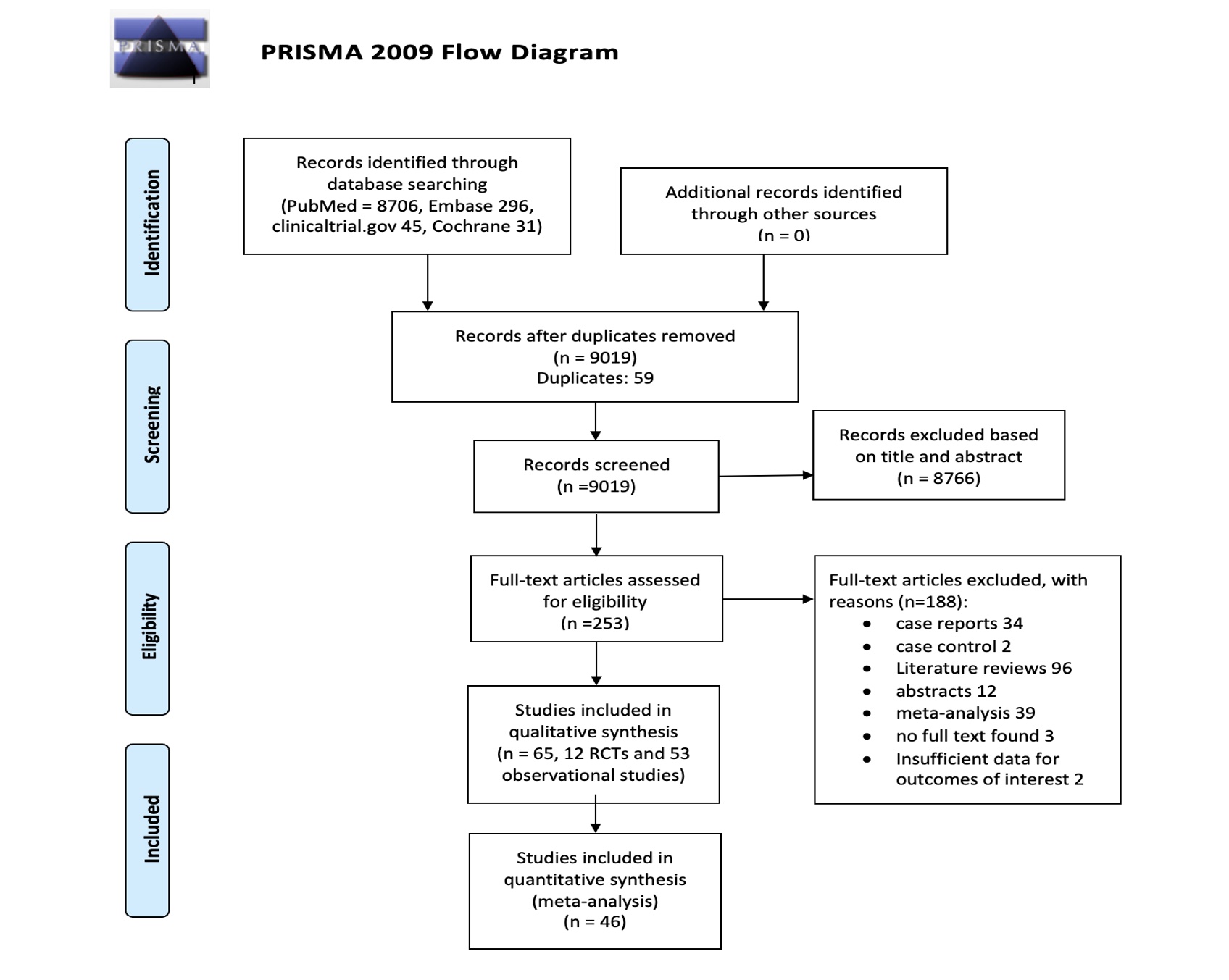
Methods: We systematically searched Medline, Embase, Clinicaltrials.gov and Cochrane electronic databases, from inception of each database to 1st November 2022 (Figure 1). The studies included in the meta-analysis were randomized clinical trials (RCTs) and observational studies that evaluated a TNF-α inhibitor (adalimumab, certolizumab pegol, etanercept, golimumab, or infliximab) as treatment for adults ( >18 years of age) with RA and reported cancer occurrence during the study period. All doses of TNF-α inhibitors were included, as well as patients on concomitant conventional DMARDs. Risk of bias was assessed using the Revised Cochrane risk-of-bias tool (RoB 2.0) for RCTs, whereas quality of the observational studies was assessed using the Newcastle Ottawa scale (NOS). Each study was evaluated for risk of bias by two separate reviewers, and a third reviewer adjudicated the results for studies where there was disagreement in the risk of bias assessment. Random effects model was employed when the number of studies was greater than 10. Between-study heterogeneity was assessed using I2 statistics. Publication bias was assessed using the Eggers p-value. Odds ratio (OR) of risk of cancer with TNF inhibitors along with their 95% confidence interval (CI) was calculated using SPSS.
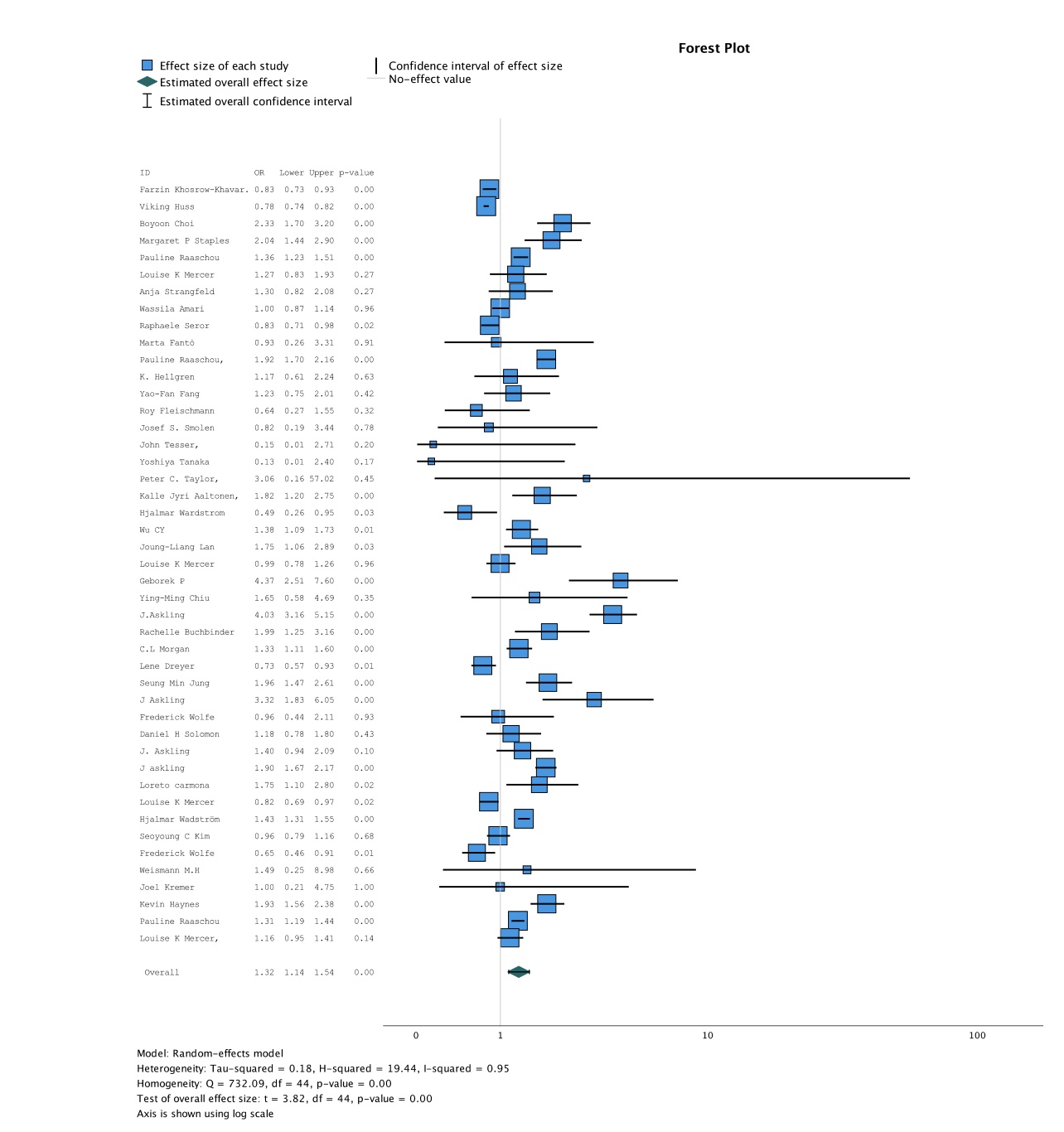
Results: 65 studies (12 RCT and 43 observational studies) met the inclusion criteria, out of which 46 studies were eligible for the meta-analysis. Remaining 19 studies had to be excluded from the meta-analysis as they were single-arm studies and hence lacked a control group. One RCT was excluded from the final analysis due to a concern of bias. The included studies had a total of 420697 patients with RA on TNF-α inhibitors, with a total of 10,329 reported cancers. Mean patient age was 55.9 years, with duration of follow up ranging from 16 weeks to 9 years. Etanercept and Adalimumab were the most frequently studied TNF-α inhibitors. Figure 2 shows that patients with RA being treated with TNF-α inhibitors had a significantly increased risk of cancer overall, compared to those on non-TNF-α inhibitors (OR 1.32, 95% CI 1.14 to 1.54; p< 0.001; I2=0.95). Subgroup analysis comparing patients on either Etanercept (OR 1.37, 95% CI 1.26 to 1.50; p< 0.001; I2=0.71) or Adalimumab (OR 1.46, 95% CI 1.29 to 1.66; p< 0.001; I2=0.67) versus those on non- TNF-α inhibitors also showed a statistically significant increase in the risk of cancer.
Conclusion: Our study shows that patients with RA on TNF-α inhibitors are at an increased risk of cancer compared to those on non- TNF-α inhibitors which includes both conventional and other biological DMARDs.
Abbreviations: OR, odds ratio; CI, confidence interval
To cite this abstract in AMA style:
Khan O, Baqir S, Naeem A, khan M, Shyam T, Slobodyanyuk K, Slobodnick A. Risk of Malignancy with TNF-α Inhibitors in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/risk-of-malignancy-with-tnf-%ce%b1-inhibitors-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-malignancy-with-tnf-%ce%b1-inhibitors-in-patients-with-rheumatoid-arthritis/